All Photos(1)
About This Item
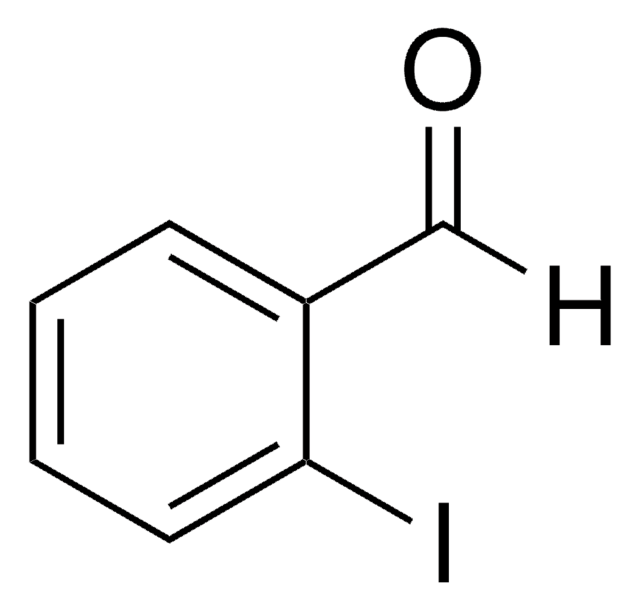
Linear Formula:
IC6H4COCH3
CAS Number:
Molecular Weight:
246.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
refractive index
n20/D 1.618 (lit.)
density
1.72 g/mL at 25 °C (lit.)
SMILES string
CC(=O)c1ccccc1I
InChI
1S/C8H7IO/c1-6(10)7-4-2-3-5-8(7)9/h2-5H,1H3
InChI key
XDXCBCXNCQGZPG-UHFFFAOYSA-N
General description
2′-Iodoacetophenone is a halogenated aromatic ketone.
Application
2′-Iodoacetophenone (2-Iodoacetophenone) may be used in the synthesis of:
- indene derivatives
- di-(o-acetylphenyl)acetylene
- indenol derivative
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kuo-Jui Chang et al.
The Journal of organic chemistry, 69(14), 4781-4787 (2004-07-03)
An efficient cobalt-catalyzed carbocylization for the synthesis of indenols and indenes and a new method for reductive decyanation are described. 2-Iodophenyl ketones and aldehydes 1a-g undergo carbocyclization with various disubstituted alkynes 2a-k in the presence of Co(dppe)I(2) and zinc powder
Charles P Casey et al.
Beilstein journal of organic chemistry, 1(1), 18-18 (2006-03-18)
The reaction of di-(o-acetylphenyl)acetylene (1) with excess dimethyl acetylenedicarboxylate (DMAD) produced bis-DMAD adducts meso-3 and rac-3. This transformation is suggested to involve thermal rearrangement of 1 to the intermediate 3,3'-dimethyl-1,1'-biisobenzofuran (A), and subsequent Diels-Alder cycloadditions of two equivalents of DMAD
Cobalt-Catalyzed Carbocyclization of o-Iodobenzaldehydes and o-Iodophenylketones with Alkynes.
Chang KJ, et al.
Organic Letters, 5(21), 3963-3966 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service