52927
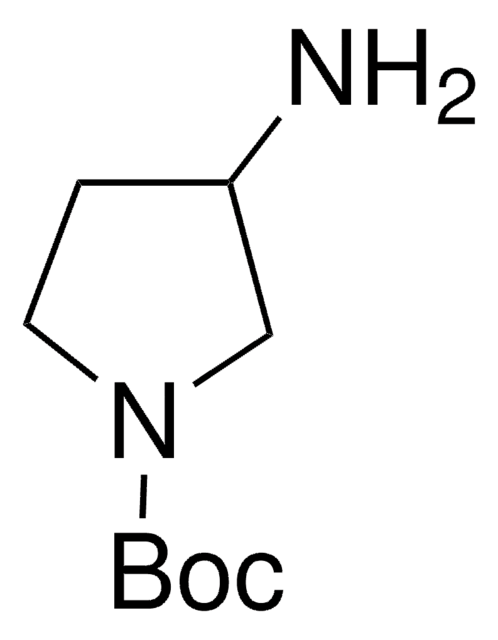
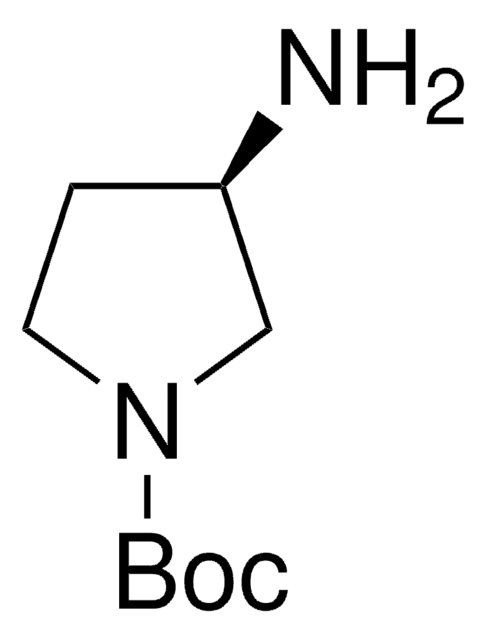
(S)-3-(Boc-amino)pyrrolidine
≥98.0% (TLC)
Synonym(s):
tert-Butyl (S)-3-pyrrolidinylcarbamate
About This Item
Recommended Products
assay
≥98.0% (TLC)
form
solid
optical activity
[α]/D -21.5±2.0°, c = 1 in ethanol
SMILES string
CC(C)(C)OC(=O)N[C@H]1CCNC1
InChI
1S/C9H18N2O2/c1-9(2,3)13-8(12)11-7-4-5-10-6-7/h7,10H,4-6H2,1-3H3,(H,11,12)/t7-/m0/s1
InChI key
DQQJBEAXSOOCPG-ZETCQYMHSA-N
Application
- 2,4,6-trisubstitued pyrido[3,4-d]pyrimidine derivatives as potent inhibitors against EGFR tyrosine kinase.
- Aminopyrrolidine scaffolds for asymmetric Morita−Baylis-Hillman reaction.
- N-benzyl-3-sulfonamidopyrrolidines as potent bacterial cell division inhibitors.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service