479179
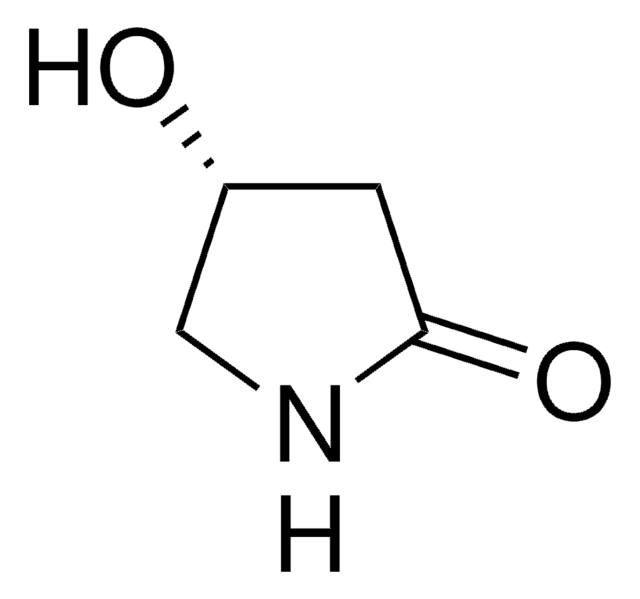
(S)-(−)-4-Hydroxy-2-pyrrolidinone
97%
Synonym(s):
(S)-β-Hydroxy-γ-butyrolactam
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7NO2
CAS Number:
Molecular Weight:
101.10
Beilstein/REAXYS Number:
1524192
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
optical activity
[α]23/D −43°, c = 1 in ethanol
mp
156-159 °C (lit.)
SMILES string
O[C@@H]1CNC(=O)C1
InChI
1S/C4H7NO2/c6-3-1-4(7)5-2-3/h3,6H,1-2H2,(H,5,7)/t3-/m0/s1
InChI key
IOGISYQVOGVIEU-VKHMYHEASA-N
Related Categories
General description
4-Hydroxy-2-pyrrolidinone is an important building block found in many bioactive compounds like streptopyrrolidine. It can be used as an intermediate in the synthesis of various γ-amino acids (GABA) and substituted 2-pyrrolidinones like cynometrine and cynodine.
Application
(S)-(−)-4-Hydroxy-2-pyrrolidinone can be used as a starting material in the preparation of:
- Biologically significant pyrrolo[1,2:1′,2′]azepino[5,6-b]indole derivatives.
- Substituted azepanes by reacting with various aldehydes via photochemical [5+2] cycloaddition.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Streptopyrrolidine, an angiogenesis inhibitor from a marine-derived Streptomyces sp. KORDI-3973
Shin HJ, et al.
Phytochemistry, 69(12), 2363-2366 (2008)
A Photochemical Two-Step Formal [5+ 2] Cycloaddition: A Condensation-Ring-Expansion Approach to Substituted Azepanes
Thullen SM, et al.
Synlett, 28(20), 2755-2758 (2017)
First synthesis of pyrrolo [1, 2: 1′, 2′] azepino [5, 6-b] indole derivatives
Perron J, et al.
Tetrahedron Letters, 44(35), 6553-6556 (2003)
G Di Silvestro et al.
Journal of pharmaceutical sciences, 82(7), 758-760 (1993-07-01)
The phase diagram of (R)- and (S)-4-hydroxy-2-pyrrolidone presents a conglomerate in the racemic mixture. The enthalpy of melting extrapolated by the Schröder-van Laar-Le Chatelier equation [change in enthalpy (delta H) = 28410 J/mol; melting temperature (TA) = 429.9 K; solidus
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service