All Photos(1)
About This Item
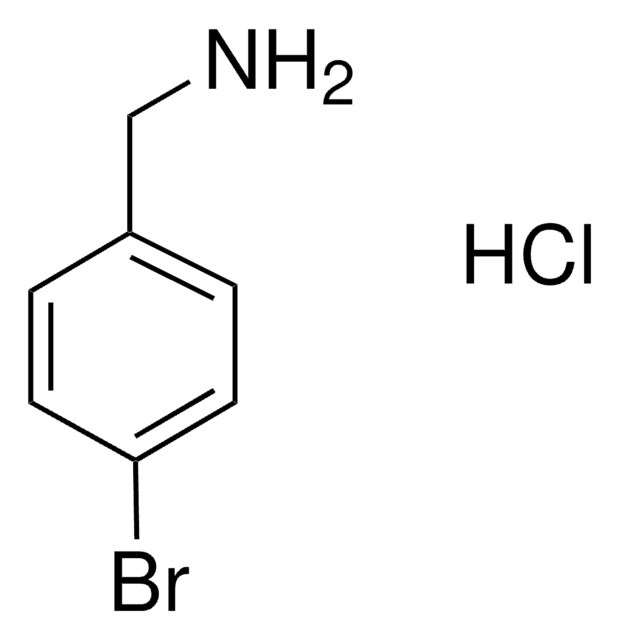
Linear Formula:
BrC6H4CH2NH2
CAS Number:
Molecular Weight:
186.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
96%
bp
110-112 °C/30 mmHg (lit.)
mp
25 °C (lit.)
density
1.473 g/mL at 25 °C (lit.)
functional group
amine
bromo
SMILES string
NCc1ccc(Br)cc1
InChI
1S/C7H8BrN/c8-7-3-1-6(5-9)2-4-7/h1-4H,5,9H2
InChI key
XRNVSPDQTPVECU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Bromobenzylamine (BBA), also known as p-bromobenzylamine, is an aryl bromide. The selective formation of nitrile or imine from BBA in the presence of red copper has been reported. The formal [4+4] reaction of BBA to form 2,6,9-triazabicyclo[3.3.1]nonane derivatives has been investigated.
Application
4-Bromobenzylamine (p-Bromobenzylamine) may be used to synthesize 7-[(p-bromobenzyl)ureido]-7,8-dihydro-α-bisabolene.
It may be used to synthesize the following 4-biphenylmethylamine derivatives:
It may be used to synthesize the following 4-biphenylmethylamine derivatives:
- (4′-fluoro-4-biphenyl)methylamine
- (4′-methoxy-4-biphenyl)methylamine
- (2′-methoxy-4-biphenyl)methylamine
- (3′-cyano-4-biphenyl)methylamine
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
>230.0 °F - closed cup
flash_point_c
> 110 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nitrogenous bisabolene sesquiterpenes from marine invertebrates.
Gulavita NK, et al.
The Journal of Organic Chemistry, 51(26), 5136-5139 (1986)
Jing Zhuang et al.
Nanoscale, 11(31), 14553-14560 (2019-07-26)
An all-inorganic CsPbI2Br perovskite with excellent phase stability and thermal stability has been considered to be a promising candidate for photovoltaic application. However, low efficiency and high moisture sensitivity hinder its advancement. In this work, we exploit 4-bromobenzylamine hydriodate post-treatment
Jiaqing Wang et al.
Chemical communications (Cambridge, England), 50(42), 5637-5640 (2014-04-16)
A novel, efficient, convenient and environmentally friendly approach for the synthesis of nitriles and imines from primary amines has been developed. Using commercially available red copper as the catalyst, ammonium bromide as the co-catalyst and molecular oxygen as the sole
Silvia Galiano et al.
Bioorganic & medicinal chemistry, 15(11), 3896-3911 (2007-04-05)
We have designed and synthesized two novel series of MCH-R1 antagonists based on a substituted biphenylmethyl urea core. SAR was explored, suggesting that optimal binding with the receptor was achieved when the biphenylmethyl group and the linker were substituted on
Ho Yeon Nam et al.
Biopolymers, 106(1), 82-88 (2015-09-26)
We developed a new method for modifying the side chains of peptoids on a solid phase resin, employing the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Optimized conditions using Pd(PPh3 )4 and K2 CO3 in the presence of Buchwald's SPhos ligand provided a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service