All Photos(1)
About This Item
Linear Formula:
(C6H5CH2O)2CO
CAS Number:
Molecular Weight:
242.27
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
bp
180-190 °C/2 mmHg (lit.)
mp
29-33 °C (lit.)
SMILES string
O=C(OCc1ccccc1)OCc2ccccc2
InChI
1S/C15H14O3/c16-15(17-11-13-7-3-1-4-8-13)18-12-14-9-5-2-6-10-14/h1-10H,11-12H2
InChI key
PIZLBWGMERQCOC-UHFFFAOYSA-N
General description
Dibenzyl carbonate (DBC) is commonly used as a benzylating agent. Dimethyl carbonate and excess of benzyl alcohol undergoes transesterification in the presence of CsF/α-Al2O3 (cesium fluoride/aluminum oxide) to form DBC. The formation of N,N-dibenzyl derivatives by the reaction of primary aliphatic amines with DBC in the presence of phosphonium salts has been investigated.
Application
Dibenzyl carbonate may be used in the synthesis of the following via benzylation reaction:
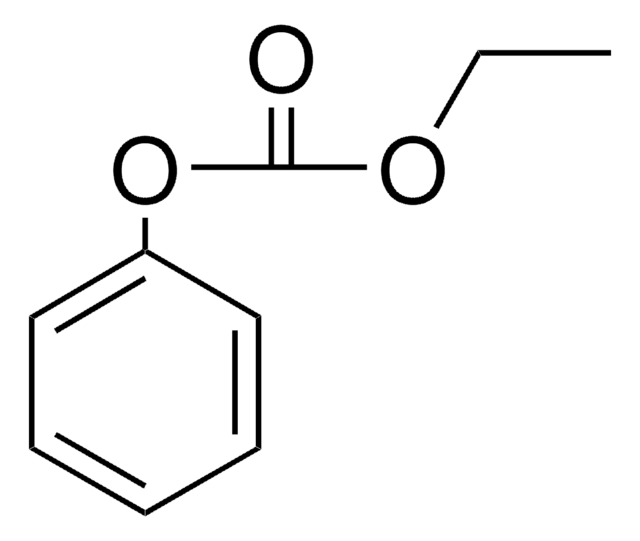
- Benzyl phenyl ether from phenol.
- 2,3-Diphenylpropionitrile from phenylacetonitrile.
- Benzyl 2,3-diphenylpropionate from benzyl phenyl acetate.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective N,N-dibenzylation of primary aliphatic amines with dibenzyl carbonate in the presence of phosphonium salts.
Loris A, et al.
The Journal of Organic Chemistry, 69(11), 3953-3956 (2004)
Selective mono-benzylation of methylene active compounds with dibenzyl carbonate: benzylation of phenol.
AlbertoaMarques C.
Journal of the Chemical Society. Perkin Transactions 1, 15, 1889-1893 (1995)
Synthesis of dibenzyl carbonate: towards a sustainable catalytic approach.
Fiorani G and Selva M.
Royal Society of Chemistry Advances, 4(4), 1929-1937 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service