All Photos(1)
About This Item
Empirical Formula (Hill Notation):
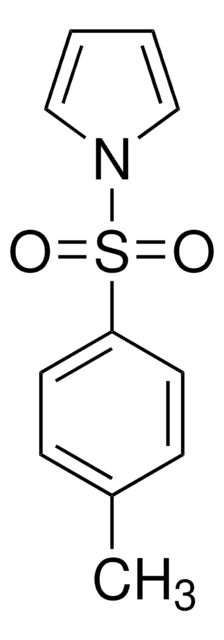
C10H9NO2S
CAS Number:
Molecular Weight:
207.25
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
solid
mp
88-91 °C (lit.)
SMILES string
O=S(=O)(c1ccccc1)n2cccc2
InChI
1S/C10H9NO2S/c12-14(13,11-8-4-5-9-11)10-6-2-1-3-7-10/h1-9H
InChI key
PPPXRIUHKCOOMU-UHFFFAOYSA-N
General description
1-(Phenylsulfonyl)pyrrole is a heterocyclic building block. 1-(Phenylsulfonyl) group serves as N-blocking and directing group in various organic syntheses.
Application
1-(Phenylsulfonyl)pyrrole (1-phenylsulfonyl-1H-pyrrole) may be used in the synthesis of 1-(phenylsulfonyl)pyrrole-2-boronic acid, via lithiation of 1-(phenylsulfonyl)-pyrrole. It may be used for the synthesis of 1-phenylsulfonyl-1H-pyrrole-3-sulfonyl chloride derivatives, which affords sulfonamide derivatives by reaction with nitrogen nucleophiles.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Efficient sulfonation of 1-phenylsulfonyl-1H-pyrroles and 1-phenylsulfonyl-1H-indoles using chlorosulfonic acid in acetonitrile.
Janosik T, et al.
Tetrahedron, 62(8), 1669-1707 (2006)
Pyrrole chemistry. XXVIII. Substitution reactions of 1-(phenylsulfonyl) pyrrole and some derivatives.
Anderson HJ, et al.
Canadian Journal of Chemistry, 63(4), 896-902 (1985)
Synthesis of 2-Aryl-1-(phenylsulfonyl) pyrroles.
Grieb JG and Ketcha DM.
Synthetic Communications, 25(14), 2145-2153 (1995)
Papireddy Kancharla et al.
The Journal of organic chemistry, 79(23), 11674-11689 (2014-11-08)
Facile and highly efficient synthetic routes for the synthesis of (S)- and (R)-23-hydroxyundecylprodiginines ((23S)-2, and (23R)-2), 23-ketoundecylprodiginine (3), and deuterium-labeled 23-hydroxyundecylprodiginine ([23-d]-2) have been developed. We demonstrated a novel Rieske oxygenase MarG catalyzed stereoselective bicyclization of (23S)-2 to premarineosin A
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service