All Photos(2)
About This Item
Linear Formula:
(CH3)3CC6H3(=O)2
CAS Number:
Molecular Weight:
164.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
solid
mp
54-58 °C (lit.)
SMILES string
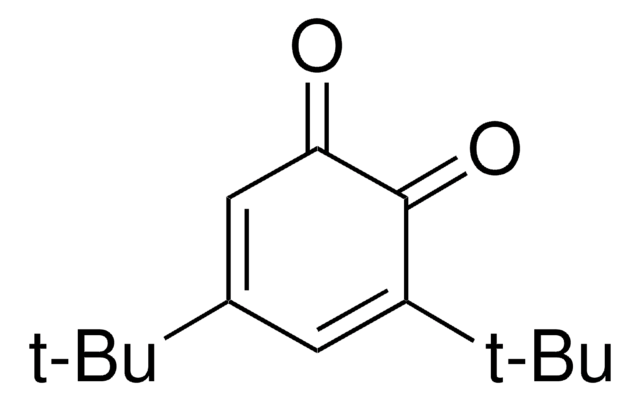
CC(C)(C)C1=CC(=O)C=CC1=O
InChI
1S/C10H12O2/c1-10(2,3)8-6-7(11)4-5-9(8)12/h4-6H,1-3H3
InChI key
NCCTVAJNFXYWTM-UHFFFAOYSA-N
General description
2-tert-Butyl-1,4-benzoquinone (TBQ, TBBQ, tBQ, BuBQ, BQ , tert-butyl-p-quinone) is a 1,4-benzoquinone derivative. It is a major metabolite of the food additive, butylated hydroxyanisole (BHA). TBQ is reported to be strongly cytotoxic in human monocytic leukemia U937 cells. TBQ is an oxidation product of 2-tert-butylhydroquinone (TBHQ). Studies confirm that TBQ induces apoptosis and cell proliferation inhibition in chronic myelogenous leukemia (CML) cells. Its binding interactions with lysozyme has been examined and found to be intermediate between BHA and TBHQ. It has been reported to be synthesized by the titanium superoxide catalyzed oxidation of 2-tert-butylphenol using aq. 30% H2O2. TBQ is one of the main neoformed compounds from TBHQ decomposition in PLA-TBHQ film (Poly lactic acid).
Application
2-tert-Butyl-1,4-benzoquinone may be used in the synthesis of azatrioxa[8]circulene.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P A Schilderman et al.
Carcinogenesis, 16(3), 507-512 (1995-03-01)
The food additive butylated hydroxyanisole (BHA) has been shown to induce gastrointestinal hyperplasia in rodents by an unknown mechanism. The relevance of this observation for human risk assessment is not clear. We therefore analysed the effect of BHA and its
Azatrioxa [8] circulenes: Planar Anti-Aromatic Cyclooctatetraenes.
Nielsen CB, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 19(12), 3898-3904 (2013)
C G Rogers et al.
Mutation research, 299(1), 9-18 (1993-03-01)
tert.-Butyl-p-quinone (TBQ), a major metabolite of the phenolic antioxidant tert.-butyl-4-hydroxyanisole (BHA), was examined for cytotoxic and genotoxic properties in an in vitro assay system with Chinese hamster V79 cells and in diploid strain D7 of Saccharomyces cerevisiae. TBQ was prepared
J H Chung et al.
Toxicology and applied pharmacology, 124(1), 123-130 (1994-01-01)
Quinone reductase (QR), in the presence of suitable substrate, results in the regeneration of NAD+ from NADH. To test the hypothesis that QR can play a role in ethanol metabolism and toxicity, we studied the effect of a quinone as
M Mizuno et al.
Mutation research, 176(2), 179-184 (1987-02-01)
The reaction products from butylated hydroxyanisole treated with nitrite under acidic conditions were investigated for mutagenic activity in Salmonella typhimurium his reversion assay and for DNA-damaging activity using H17 Rec+ (wild) and M45 Rec- (recombinationless) of Bacillus subtilis. The chloroform
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service