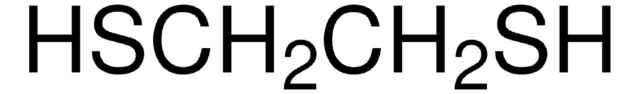398020
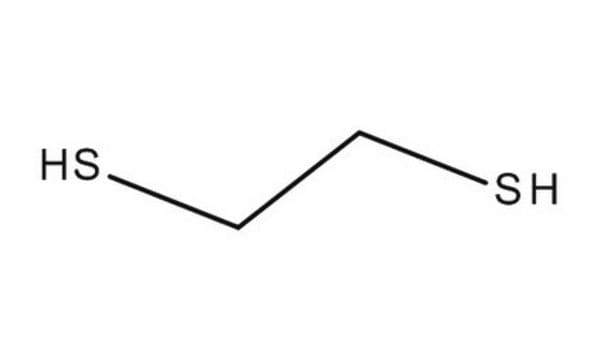
1,2-Ethanedithiol
technical grade, ≥90%
Synonym(s):
1,2-Dimercaptoethane, Dithioglycol, Ethylene mercaptan
About This Item
Recommended Products
grade
technical grade
Quality Level
vapor density
>1 (vs air)
vapor pressure
4.8 mmHg ( 20 °C)
assay
≥90%
form
liquid
refractive index
n20/D 1.558 (lit.)
bp
144-146 °C (lit.)
mp
−41 °C (lit.)
density
1.123 g/mL at 25 °C (lit.)
SMILES string
SCCS
InChI
1S/C2H6S2/c3-1-2-4/h3-4H,1-2H2
InChI key
VYMPLPIFKRHAAC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis of membrane-permeant, fluorogenic biarsenicals.
- Development of microarrays of protein interaction and quantifying the domain-peptide interactions in high throughput using fluorescently labeled synthetic peptides.
- As insolubilizing agent in a study to evaluate the structural, optical, and electrical properties of high-quality films of PbSe nanocrystals fabricated by a layer-by-layer (LbL) dip-coating method.
- Quantitation of the extent of protein phosphorylation using a phosphoprotein isotope-coded affinity tag (PhIAT).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
111.2 °F - closed cup
flash_point_c
44 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service