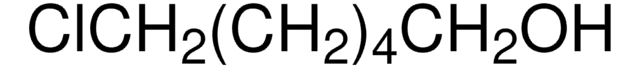All Photos(1)
About This Item
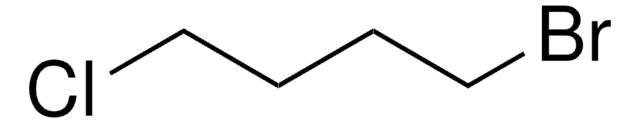
Linear Formula:
I(CH2)4Cl
CAS Number:
Molecular Weight:
218.46
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
liquid
contains
copper as stabilizer
refractive index
n20/D 1.54 (lit.)
bp
88-89 °C/19 mmHg (lit.)
density
1.785 g/mL at 25 °C (lit.)
functional group
chloro
iodo
SMILES string
ClCCCCI
InChI
1S/C4H8ClI/c5-3-1-2-4-6/h1-4H2
InChI key
JXOSPTBRSOYXGC-UHFFFAOYSA-N
General description
1-Chloro-4-iodobutane is a halogenated hydrocarbon. It is an α,ω-dihaloalkane and undergoes electrogenerated Nickel(I) salen (N,N′-bis(salicylidene)ethylenediamine) catalyzed reduction to afford 1,8-dichlorooctane. Electrochemical reduction of 1-chloro-4-iodobutane at glassy carbon cathode has been investigated by cyclic voltammetry and controlled-potential electrolysis.
Application
1-Chloro-4-iodobutane may be used in the following studies:
- Preparation of 6-hendecenoic acid.
- Catalytic asymmetric synthesis of levobupivacaine.
- Synthesis of alkaloids such as deoxyvasicinone, mackinazolinone.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
199.4 °F - closed cup
flash_point_c
93 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Studies directed towards asymmetric synthesis of levobupivacaine.
Kumar S and Ramachandran U.
Tetrahedron Letters, 46(1), 19-21 (2005)
The synthesis of unsaturated fatty acids.
K AHMAD et al.
Journal of the American Chemical Society, 70(5), 1699-1699 (1948-05-01)
W Russell Bowman et al.
Organic & biomolecular chemistry, 5(1), 103-113 (2006-12-14)
Alkyl, aryl, heteroaryl and acyl radicals have been cyclised onto the 2-position of 3H-quinazolin-4-one. The side chains containing the radical precursors were attached to the nitrogen atom in the 3-position. The cyclisations take place by aromatic homolytic substitution hence retain
Keivan Sadrerafi et al.
Drug design, development and therapy, 12, 987-995 (2018-05-08)
Our previous study indicated that carborane containing small-molecule 1-(hydroxymethyl)-7-(4'-(trans-3″-(3'″-pyridyl)acrylamido)butyl)-1,7-dicarbadodecaborane (hm-MC4-PPEA), was a potent inhibitor of nicotinamide phosphoribosyltransferase (Nampt). Nampt has been shown to be upregulated in most cancers and is a promising target for the treatment of many different types
Homogeneous catalytic reduction of a, ?-dihaloalkanes with electrogenerated nickel (I) salen.
Mubarak MS and Peters DG.
Journal of Electroanalytical Chemistry, 388(1), 195-198 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service