393851
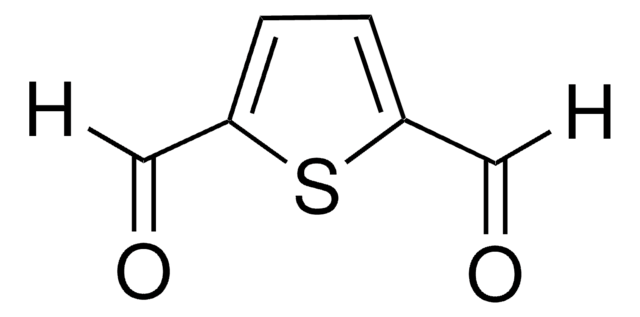
2-Hydroxy-5-methyl-1,3-benzenedicarboxaldehyde
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H2(CH3)(CHO)2
CAS Number:
Molecular Weight:
164.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
solid
mp
128-130 °C (lit.)
functional group
aldehyde
SMILES string
Cc1cc(C=O)c(O)c(C=O)c1
InChI
1S/C9H8O3/c1-6-2-7(4-10)9(12)8(3-6)5-11/h2-5,12H,1H3
InChI key
ZBOUXALQDLLARY-UHFFFAOYSA-N
General description
2-Hydroxy-5-methyl-1,3-benzenedicarboxaldehyde (2-hydroxy-5-methylisophthalaldehyde) is a dialdehyde derivative. It has been synthesized by heating p-cresol with hexamethylenetetramine. The structure has been confirmed by 1H and 13C NMR. It is an important raw material for the synthesis of various binucleating schiff base ligand.
Application
2-Hydroxy-5-methyl-1,3-benzenedicarboxaldehyde (2-hydroxy-5-methylisophthalaldehyde) is suitable reagent used in the synthesis of 2-(2′-vinyloxyethoxy)-5-methylisophthaldehyde and chiral calixsalen macrocycles.
2-Hydroxy-5-methyl-1,3-benzenedicarboxaldehyde may be used in the synthesis of the following:
2-Hydroxy-5-methyl-1,3-benzenedicarboxaldehyde may be used in the synthesis of the following:
- 3-[(2,4-Dichlorophenyl)iminomethyl]-2-hydroxy-5-methylbenzaldehyde, a Schiff base.
- 2-Hydroxy-3-methoxymethyl-5-methylbenzaldehyde.
- Acyclic Schiff-base ligands.
- Macrobicyclic ligands (MSB).
- 4-Methyl-2,6-divinylphenol.
- 2-Hydroxy-3-dimethoxymethyl-5-methylbenzaldehyde.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bimetallic copper (II) and zinc (II) complexes of acyclic Schiff base ligands derived from amino acids.
Arbaoui A, et al
Inorgorganica Chimica Acta, 365(1), 96-102 (2011)
Binuclear metal complexes. 1. Dicopper (II) complexes with binucleating ligands derived from 2-hydroxy-5-methylisophthalaldehyde and 2-(2-aminoethyl) pyridine or histamine.
Grzybowski JJ, et al
Inorganic Chemistry, 17(11), 3078-3082 (1978)
New efficient ruthenium metathesis catalyst containing chromenyl ligand.
Hryniewicka A, et al
Journal of Organometallic Chemistry, 695(9), 1265-1270 (2010)
3-[(2, 4-Dichlorophenyl) iminomethyl]-2-hydroxy-5-methylbenzaldehyde.
Kilic I, et al
Acta Crystallographica Section E, Structure Reports Online, 65(6), 1347-1347 (2009)
Steric effect in the free radical polymerization of vinyl ethers containing electron-deficient olefin groups.
Lee JY and Jin MK
Polymer Bull., 44(3), 2777-2284 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service