392510
1-Iodo-3,5-dimethylbenzene
99%
Synonym(s):
5-Iodo-m-xylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2C6H3I
CAS Number:
Molecular Weight:
232.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.594 (lit.)
bp
92-94 °C/3 mmHg (lit.)
density
1.608 g/mL at 25 °C (lit.)
functional group
iodo
SMILES string
Cc1cc(C)cc(I)c1
InChI
1S/C8H9I/c1-6-3-7(2)5-8(9)4-6/h3-5H,1-2H3
InChI key
ZLMKEENUYIUKKC-UHFFFAOYSA-N
Related Categories
General description
1-Iodo-3,5-dimethylbenzene (5-Iodo-m-xylene) is an aryl halide. It can be obtained from 5-bromo-m-xylene, via copper-catalyzed halogen exchange reaction, in the presence of NaI or KI in n-BuOH or DMF (solvents). It undergoes reaction with phenol in the presence of CuFe2O4 nano powder as a recyclable catalyst to afford 1,3-dimethyl-5-phenoxybenzene.
Application
1-Iodo-3,5-dimethylbenzene (5-iodo-m-xylene) is suitable for use in the synthesis of N-(3,5-xylyl)-N-ethylaniline, an arylamine.
It may be used in the following studies:
It may be used in the following studies:
- α-Arylation of ketones.
- Copper-catalyzed N-arylation of imidazoles.
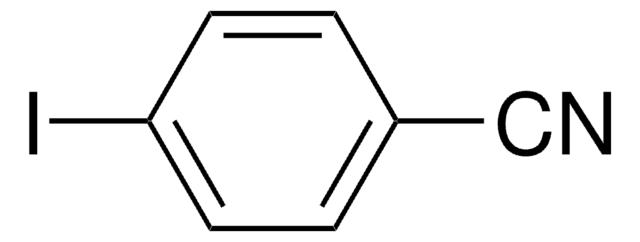
- Cyanation of 5-iodo-m-xylene to form 3,5-dimethylbenzonitrile.
- Synthesis of 1,3-Dimethyl-5-phenoxybenzene by nano-CuFe2O4 catalyzed C-O cross-coupling with phenol.
- CuBr-catalyzed amination of 1-iodo-3,5-dimethylbenzene to form N-Allyl-3,5-dimethylbenzenamine.
- Copper-catalyzed C-S bond-formation between 5-iodo-m-xylene and thiophenol.
- As a starting material in the synthesis of biphenyl-3,3′,5,5′-tetracarboxylic acid.
- Radical bromination of 5-iodo-m-xylene by N-bromosuccinimide to form 1,3-bis(bromomethyl)-5-iodobenzene.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
On the synthesis of heterocyclic dendrons.
Diez-Barra E, et al.
ARKIVOC (Gainesville, FL, United States), 2002(5), 17-25 (2002)
Fuk Yee Kwong et al.
Organic letters, 4(20), 3517-3520 (2002-09-27)
An efficient copper-catalyzed carbon-sulfur bond formation reaction was developed. This method is particularly noteworthy given its experimental simplicity, high generality, and exceptional level of functional group toleration and the low cost of the catalyst system. [reaction: see text]
M H Ali et al.
The Journal of organic chemistry, 66(8), 2560-2565 (2001-04-17)
Aryl iodides are coupled with amines to give the corresponding arylamines in high yield in the presence of palladium, a suitable ligand, and NaOt-Bu. Functionalized aryl iodides give good yields of the corresponding arylamines when Cs(2)CO(3) is substituted as the
Recyclable and reusable nano-CuFe2O4 catalyzed CO cross-coupling.
Avudoddi V, et al.
European Journal of Chemistry, 3(3), 298-304 (2012)
Jacopo Zanon et al.
Journal of the American Chemical Society, 125(10), 2890-2891 (2003-03-06)
An efficient copper-catalyzed domino halogen exchange-cyanation procedure for aryl bromides was developed utilizing 10 mol % CuI, 20 mol % KI, 1.0 equiv of the inexpensive N,N'-dimethylethylenediamine as ligand, and 1.2 equiv of NaCN in toluene at 110 degrees C.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service