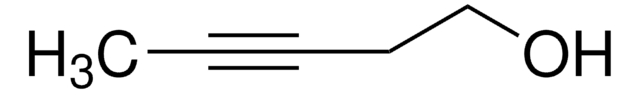All Photos(1)
About This Item
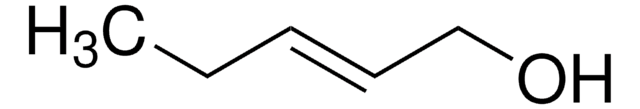
Linear Formula:
C2H5C≡CCH2OH
CAS Number:
Molecular Weight:
84.12
Beilstein/REAXYS Number:
1734007
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.452 (lit.)
bp
84-85 °C/57 mmHg (lit.)
density
0.909 g/mL at 25 °C (lit.)
SMILES string
CCC#CCO
InChI
1S/C5H8O/c1-2-3-4-5-6/h6H,2,5H2,1H3
InChI key
WLPYSOCRPHTIDZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Pentyn-1-ol was employed as starting reagent for the synthesis of (-)-muricatacin. It was also used in the preparation of (2Z)-3-tributylstannyl-2-penten-1-ol.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
136.4 °F - closed cup
flash_point_c
58 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Wada et al.
Chemical & pharmaceutical bulletin, 48(9), 1391-1394 (2000-09-19)
Palladium catalyzed cross coupling reactions of a vinyl triflate intermediate and various alkenyl stannanes afforded trisubstituted Z-olefins stereoselectively in high yields. These olefins were then converted to the corresponding 9Z-retinoic acids via Horner-Emmons reaction and subsequent basic hydrolysis in excellent
H Makabe et al.
Bioscience, biotechnology, and biochemistry, 57(6), 1028-1029 (1993-06-01)
The synthesis of (-)-muricatacin starting from 1-bromododecane and 2-pentyn-1-ol is described. 2-Pentadecyn-1-ol (4), which was prepared from 1-bromododecane (2) and 2-pentyn-1-ol (3), was converted to epoxy alcohol 6 through a two-step reaction sequence, 6 being successively submitted to tosylation, iodination
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service