All Photos(1)
About This Item
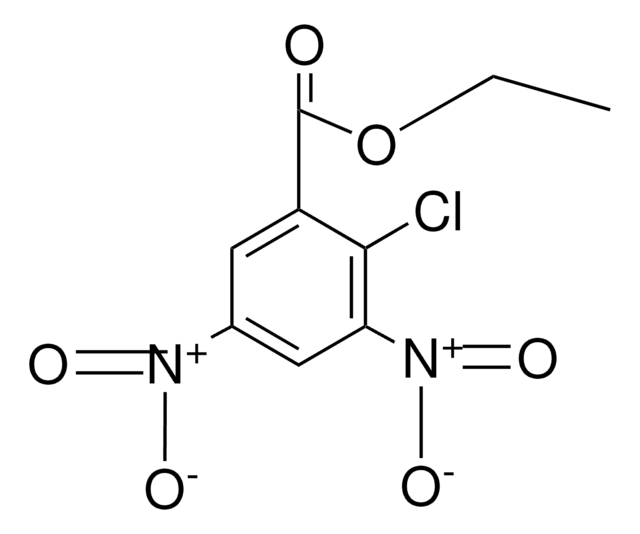
Linear Formula:
(O2N)2C6H3CO2C2H5
CAS Number:
Molecular Weight:
240.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
mp
94-95 °C (lit.)
SMILES string
CCOC(=O)c1cc(cc(c1)[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C9H8N2O6/c1-2-17-9(12)6-3-7(10(13)14)5-8(4-6)11(15)16/h3-5H,2H2,1H3
InChI key
IBQREHJPMPCXQA-UHFFFAOYSA-N
General description
Ethyl 3,5-dinitrobenzoate forms charge transfer spectra with n-alkane solutions containing hexakis(n-hexyloxy)triphenylene. Nuclear magnetic resonance spectra of ethyl 3,5-dinitrobenzoate was studied.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The mechanism of action of ethanolamine deaminase. I. Studies with isotopic hydrogen and oxygen.
B M Babior
The Journal of biological chemistry, 244(2), 449-456 (1969-01-25)
Aggregates of hexakis (n-hexyloxy) triphenylene self-assemble in dodecane solution: intercalation of (-)-menthol 3, 5-dinitrobenzoate induces formation of helical structures.
Gallivan JP and Schuster GB.
The Journal of Organic Chemistry, 60(8), 2423-2429 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service