All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H4BrNO2
CAS Number:
Molecular Weight:
226.03
Beilstein/REAXYS Number:
131211
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
mp
194-198 °C (lit.)
functional group
imide
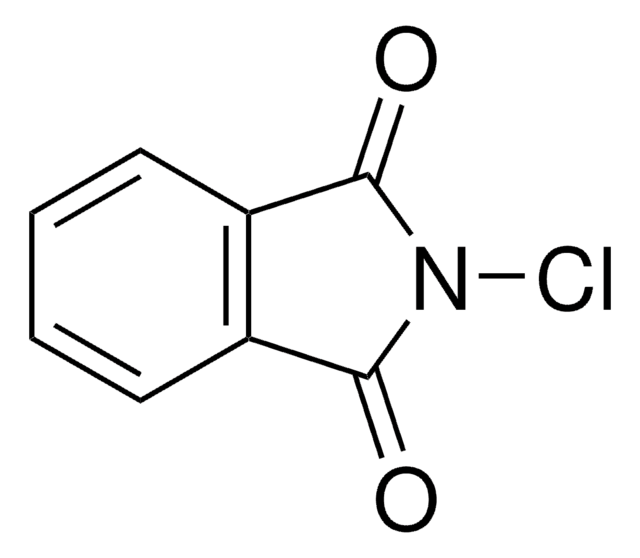
SMILES string
BrN1C(=O)c2ccccc2C1=O
InChI
1S/C8H4BrNO2/c9-10-7(11)5-3-1-2-4-6(5)8(10)12/h1-4H
InChI key
MARXMDRWROUXMD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N-Bromophthalimide has been used:
- as reagent in allylic amination reactions of alkenes
- brominating reagent in enantioselective synthesis of multisubstituted biaryl derivatives by chiral phosphoric acid catalyzed asymmetric bromination
- as a titrant in titrimetric determination of isoniazid in pure form or in tablets
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Krishnakumar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 62(4-5), 918-925 (2005-06-14)
Fourier transform infrared (FT-IR) spectra of phthalimide and N-bromophthalimide have been recorded in the range of 4000-400 cm-1. With the hope of providing more and effective information on the fundamental vibrations, a normal coordinate analysis has been performed on phthalimide
Titrimetric determination of para-aminobenzoic acid using N-bromophthalimide and N-bromosaccharin.
K G Kumar et al.
Journal of pharmaceutical and biomedical analysis, 7(5), 627-631 (1989-01-01)
Titrimetric methods for the determination of some sulpha drugs using N-bromophthalimide and N-bromosaccharin.
K G Kumar et al.
The Analyst, 113(9), 1369-1372 (1988-09-01)
A M el-Brashy et al.
Journal of pharmaceutical and biomedical analysis, 10(6), 421-426 (1992-06-01)
Two methods are proposed for the determination of isoniazid in pure form or in tablets. In the first method chlorpromazine hydrochloride, when treated with 2-iodoxybenzoic acid as an oxidant in 50% w/v o-phosphoric acid solution, is oxidized to chlorpromazine free
Tejas P Pathak et al.
Journal of the American Chemical Society, 134(14), 6120-6123 (2012-04-03)
We report the site-selective bromination of vancomycin to produce, with substantial efficiency, previously unknown monobromovancomycins, a dibromovancomycin, and a tribromovancomycin. We document the inherent reactivity of native vancomycin toward N-bromophthalimide. We then demonstrate significant rate acceleration and perturbation of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service