255254
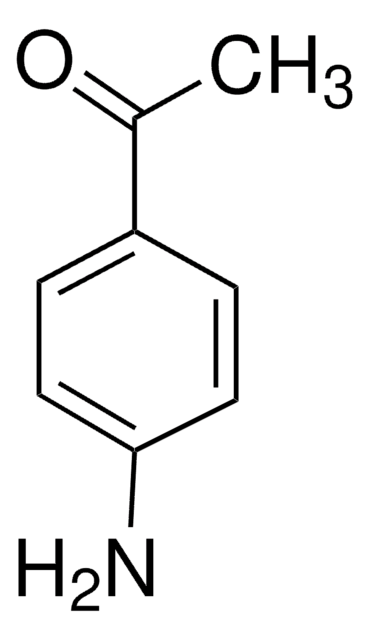
3-Aminobenzophenone
97%
Synonym(s):
3-Benzoylaniline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H4C(O)C6H5
CAS Number:
Molecular Weight:
197.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
81-84 °C (lit.)
functional group
ketone
phenyl
SMILES string
Nc1cccc(c1)C(=O)c2ccccc2
InChI
1S/C13H11NO/c14-12-8-4-7-11(9-12)13(15)10-5-2-1-3-6-10/h1-9H,14H2
InChI key
FUADXEJBHCKVBN-UHFFFAOYSA-N
General description
3-Aminobenzophenone forms cyclodextrin (α and β) based nanostructures through the supramolecular self assembly.
Application
3-Aminobenzophenone has been used in the synthesis of racemic benzophenone ureas, chiral photoaffinity labeling probes.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Rajendiran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 127, 52-60 (2014-03-19)
Cyclodextrin (α and β) based nanostructures formed with 2-aminobenzophenone, 3-aminobenzophenone through the supramolecular self assembly are studied by absorption, fluorescence, time-resolved fluorescence, SEM, TEM, FT-IR, DSC, PXRD and (1)H NMR. The unequal layer by layer nanosheets and nanoribbons are formed
Elizabeth M Hadac et al.
Journal of medicinal chemistry, 49(3), 850-863 (2006-02-03)
An understanding of the molecular basis of drug action provides opportunities for refinement of drug properties and for development of more potent and selective molecules that act at the same biological target. In this work, we have identified the active
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service