All Photos(2)
About This Item
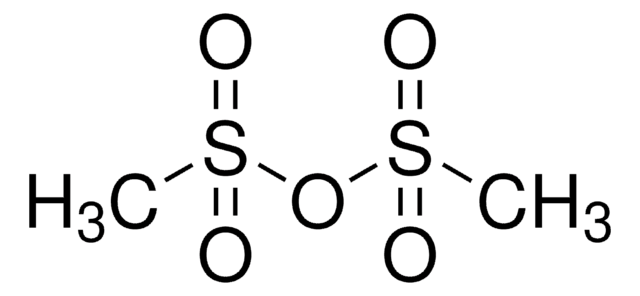
Linear Formula:
Cl3CSO2Cl
CAS Number:
Molecular Weight:
217.89
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
chunks
mp
137-140 °C (lit.)
SMILES string
ClC(Cl)(Cl)S(Cl)(=O)=O
InChI
1S/CCl4O2S/c2-1(3,4)8(5,6)7
InChI key
ZCPSWAFANXCCOT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Trichloromethanesulfonyl chloride is an efficient free radical chlorinating agent. It reacts with pent-4-enylcobaloximes in inert solvent under tugsten lamp irradiation to yield 2-(β,β,β- trichloroethyl)sulfolanes. It also reacts with trimethylsilyl enol ethers of acetophenones in the presence of a ruthenium (II) phosphine complex to yield 1-aryl-3,3-dichloropropen-1-one and α-chloroacetophenones.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of trichloromethanesulfonyl chloride and carbon tetrachloride with silyl enol ethers catalyzed by a ruthenium (II) phosphine complex.
Kamigata N, et al.
Journal of Organometallic Chemistry, 552(1), 9-43 (1998)
TRICHLOROMETHANESULFONYL CHLORIDE AS A SELECTIVE CHLORINATING AGENT1.
Huyser ES.
Journal of the American Chemical Society, 82(19), 5246-5247 (1960)
Homolytic displacement at saturated carbon. Part 9. The reactions of trichloromethanesulfonyl chloride with pent-4-enylcobaloximes and with olefins. A novel route to (trichloroethyl) sulfolanes via an SHi mechanism.
Ashcroft MR, et al.
The Journal of Organic Chemistry, 49(10), 1751-1761 (1984)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service