216879
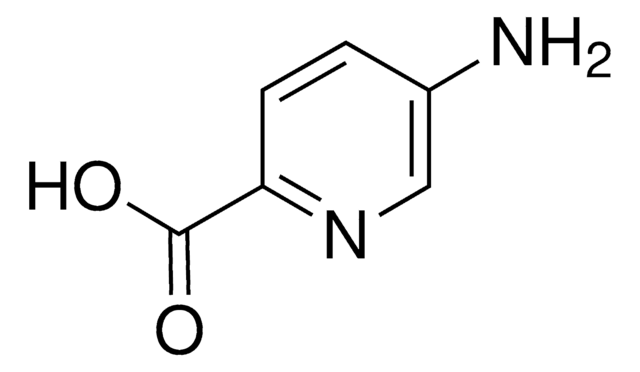
6-Aminopyridine-3-carboxylic acid
97%
Synonym(s):
6-Aminonicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O2
CAS Number:
Molecular Weight:
138.12
Beilstein/REAXYS Number:
115992
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
>300 °C (lit.)
SMILES string
Nc1ccc(cn1)C(O)=O
InChI
1S/C6H6N2O2/c7-5-2-1-4(3-8-5)6(9)10/h1-3H,(H2,7,8)(H,9,10)
InChI key
ZCIFWRHIEBXBOY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Electrosynthesis of 6-aminopyridine-3-carboxylic acid (6-aminonicotinic acid) by electrochemical reduction of 2-amino-5-bromo and 2-amino-5-chloropyridine in the presence of CO2 at silver electrode has been reported.
Application
6-Aminopyridine-3-carboxylic acid (6-Aminonicotinic acid) was used in the preparation of resin-bound 2-aminoazine.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A three-component solid-phase synthesis of 3-aminoimidazo [1, 2-a] azines.
Blackburn C.
Tetrahedron Letters, 39(31), 5469-5472 (1998)
D J Jamieson et al.
Journal of bacteriology, 168(1), 389-397 (1986-10-01)
Expression of the tripeptide permease gene tppB is anaerobically induced. This induction is independent of the fnr (oxrA) gene product, which is known to be required for the anaerobic induction of several respiratory enzymes. We isolated, characterized, and mapped mutations
Raman Sharma et al.
Xenobiotica; the fate of foreign compounds in biological systems, 49(12), 1447-1457 (2019-02-13)
1. The absorption, metabolism, and excretion of a single oral 450-mg dose of [14C]-(S)-6-(3-cyclopentyl-2-(4-trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinic acid (PF-04991532), a hepatoselective glucokinase activator, was investigated in humans. Mass balance was achieved with ∼94.6% of the administered dose recovered in urine and feces. The
J W Foster et al.
Journal of general microbiology, 130(11), 2873-2881 (1984-11-01)
Mutants of Salmonella typhimurium supersensitive to the nicotinic acid analogue 6-amino-nicotinic acid (6ANA) were isolated as unable to grow on what are normally subinhibitory concentrations of the analogue. The mutations were classified on the basis of their map positions as
Electrocatalytic synthesis of 6-aminonicotinic acid at silver cathodes under mild conditions.
Gennaro A, et al.
Electrochemical Communications, 6(7), 627-631 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service