196967
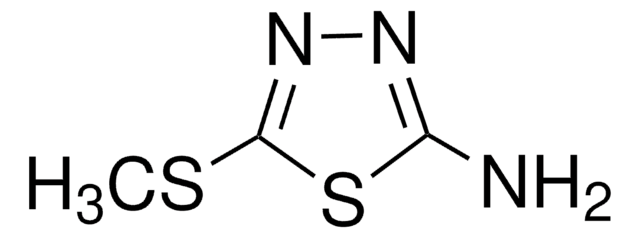
2-Amino-5-trifluoromethyl-1,3,4-thiadiazole
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H2F3N3S
CAS Number:
Molecular Weight:
169.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
225-227 °C (lit.)
solubility
methanol: soluble 0.25 g/10 mL, clear, colorless
functional group
fluoro
SMILES string
Nc1nnc(s1)C(F)(F)F
InChI
1S/C3H2F3N3S/c4-3(5,6)1-8-9-2(7)10-1/h(H2,7,9)
InChI key
LTEUXHSAYOSFGQ-UHFFFAOYSA-N
Application
2-Amino-5-trifluoromethyl-1,3,4-thiadiazole was used in the synthesis of:
- series of 2-sulfonamido/trifluoromethyl-6-(4′-substituted aryl/heteroaryl)imidazo[2,1-b]-1,3,4-thiadiazole derivatives
- 2-trifluoromethyl/sulfonamido-5,6-diarylsubstituted imidazo[2,1-b]-1,3,4-thiadiazole derivatives
- bicyclic bridgehead itrogen heterocycles
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bridgehead nitrogen heterocycles. II. Formation by reaction of. alpha.-amino nitrogen heterocyclic compounds with chlorothioformyl chloride.
Pilgram K and Skiles RD.
The Journal of Organic Chemistry, 38(8), 1575-1578 (1973)
Andanappa K Gadad et al.
Bioorganic & medicinal chemistry, 12(21), 5651-5659 (2004-10-07)
A series of 2-sulfonamido/trifluoromethyl-6-(4'-substituted aryl/heteroaryl)imidazo[2,1-b]-1,3,4-thiadiazole derivatives (II) have been synthesized by reaction of 2-amino-5-sulfonamido/trifluoromethyl-1,3,4-thiadiazoles and an appropriate alpha-haloaryl/heteroaryl ketones. Further 5-bromo (III), 5-thiocyanato (IV), 5-gaunylhydrazone (V) derivatives were synthesized in order to study the effect of these substituents on biological
Andanappa K Gadad et al.
Bioorganic & medicinal chemistry, 16(1), 276-283 (2007-10-17)
A series of 2-trifluoromethyl/sulfonamido-5,6-diarylsubstituted imidazo[2,1-b]-1,3,4-thiadiazole derivatives 15a-j have been synthesized by the reaction of 2-amino-5-trifluoromethyl/sulfonamido-1,3,4-thiadiazoles 14a-b and appropriately substituted alpha-bromo-1,2-(p-substituted)diaryl-1-ethanones 13a-h. Structures of these compounds were established by IR, (1)H NMR, (13)C NMR, Mass, and HRMS data. The selected compounds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service