193585
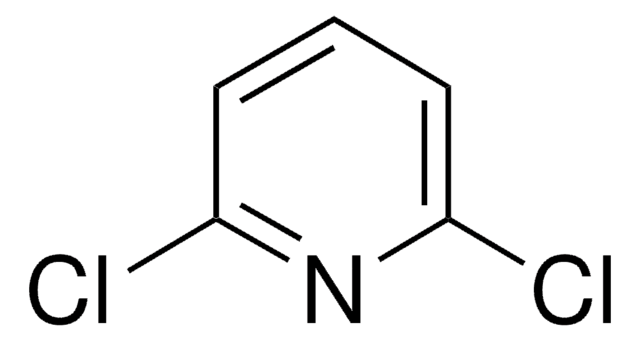
2,6-Dichloro-3-nitropyridine
technical grade, 92%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H2Cl2N2O2
CAS Number:
Molecular Weight:
192.99
Beilstein/REAXYS Number:
1619741
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39151701
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
assay
92%
mp
55-60 °C (lit.)
functional group
chloro
nitro
SMILES string
[O-][N+](=O)c1ccc(Cl)nc1Cl
InChI
1S/C5H2Cl2N2O2/c6-4-2-1-3(9(10)11)5(7)8-4/h1-2H
InChI key
SHCWQWRTKPNTEM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Dichloro-3-nitropyridine undergoes macrocyclic condensation reaction with resorcinol derivatives to yield chiral tetraoxacalix[2]arene[2]pyridines.
Application
2,6-Dichloro-3-nitropyridine was used:
- in the synthesis of pyridyldifluoroacetates
- as starting reagent in the preparation of bicyclooxacalixhetarene
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shuai Pan et al.
Organic letters, 14(24), 6254-6257 (2012-12-12)
Inherently chiral tetraoxacalix[2]arene[2]pyridines containing C(2) symmetry were synthesized efficiently from a macrocyclic condensation reaction of resorcinol derivatives with 2,6-dichloro-3-nitropyridine in a one-pot reaction manner, while tetraoxacalix[2]arene[2]pyridine with an ABCD-substitution pattern was prepared in a good yield by means of a
Wouter Maes et al.
Chemical Society reviews, 37(11), 2393-2402 (2008-10-25)
Oxacalix[n]arenes, reassessed members of the calixarene family in which the traditional methylene bridges are replaced by oxygen atoms, have emerged as a promising class of macrocycles in recent years. This tutorial review summarizes the synthetic progress made in the field
Copper-mediated reaction of 2-halopyridines with ethyl bromodifluoroacetate.
Ashwood MS, et al.
Tetrahedron Letters, 43(50), 9271-9273 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service