All Photos(1)
About This Item
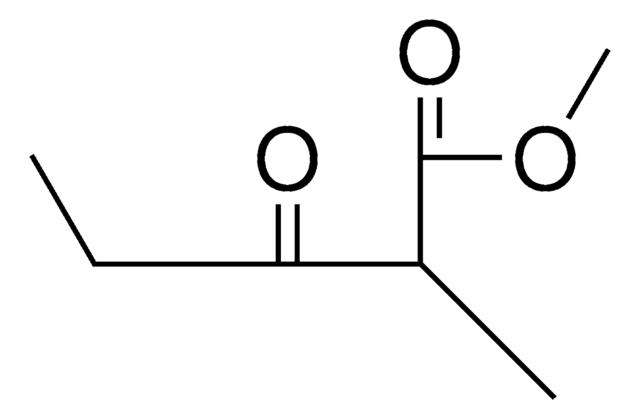
Linear Formula:
CH3COCH(C2H5)COOC2H5
CAS Number:
Molecular Weight:
158.19
Beilstein/REAXYS Number:
636286
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
90%
form
liquid
refractive index
n20/D 1.421 (lit.)
bp
87-189 °C/743 mmHg (lit.)
density
0.981 g/mL at 25 °C (lit.)
functional group
ester
ketone
SMILES string
CCOC(=O)C(CC)C(C)=O
InChI
1S/C8H14O3/c1-4-7(6(3)9)8(10)11-5-2/h7H,4-5H2,1-3H3
InChI key
OKANYBNORCUPKZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl 2-ethylacetoacetate was used in the synthesis of 2-alkylidenetetrahydrofurans, diphenyllead(IV) thiosemicarbazonates and pyrazolonates. It was used as starting reagent in the synthesis of 2-ethylfumaric acid.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
156.2 °F - closed cup
flash_point_c
69 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Diphenyllead (IV) thiosemicarbazonates and pyrazolonates: Synthesis and characterization.
Casas JS, et al.
Polyhedron, 28(5), 1029-1039 (2009)
Hans Raj et al.
Applied microbiology and biotechnology, 94(2), 385-397 (2011-10-19)
Methylaspartate ammonia lyase (MAL; EC 4.3.1.2) catalyzes the reversible addition of ammonia to mesaconate to give (2S,3S)-3-methylaspartate and (2S,3R)-3-methylaspartate as products. MAL is of considerable biocatalytic interest because of its potential use for the asymmetric synthesis of substituted aspartic acids
Efficient synthesis of functionalized furans and benzofurans based on a '[3+ 2] cyclization/oxidation'strategy.
Bellur E, et al.
Tetrahedron Letters, 46(13), 2185-2187 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service