All Photos(1)
About This Item
Linear Formula:
CH3C(OC2H5)=C(CN)2
CAS Number:
Molecular Weight:
136.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
90-92 °C (lit.)
functional group
ether
nitrile
SMILES string
CCO\C(C)=C(\C#N)C#N
InChI
1S/C7H8N2O/c1-3-10-6(2)7(4-8)5-9/h3H2,1-2H3
InChI key
BOSVWXDDFBSSIZ-UHFFFAOYSA-N
General description
(1-Ethoxyethylidene)malononitrile is also referred as 2-(1-ethoxyethylidene)malononitrile.
Application
(1-Ethoxyethylidene)malononitrile was used in the synthesis of benzyl 5-amino-4-cyano-3-methyl-1H pyrazole-1-carboxylate and 2-amino-6-mercaptopyridine-3,5-dicarbonitrile derivatives.
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Diaa A Ibrahim et al.
European journal of medicinal chemistry, 46(12), 5825-5832 (2011-10-18)
The design and synthesis of a small library of 4-aminopyrido[2,3-d]pyrimidine derivatives is reported. The potential activity of these compounds as CDK2/Cyclin A, CDK4/Cyclin D, EGFR and anti-tumor was evaluated by cytotoxicity studies in A431a, SNU638b, HCT116 and inhibition of CDK2-Cyclin
Yaojun Gao et al.
Journal of combinatorial chemistry, 12(1), 69-74 (2009-12-05)
A solid-phase synthesis of 5-aminopyrazole has been developed and applied to the preparation of pyrazolo[5,1-d][1,2,3,5]tetrazine-4(3H)-ones. In this strategy, a one-pot reaction from 5-aminopyrazoles to the pyrazolo[5,1-d][1,2,3,5]tetrazine-4(3H)-ones which provided the compounds in good yields was demonstrated. Using this synthetic strategy, we
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service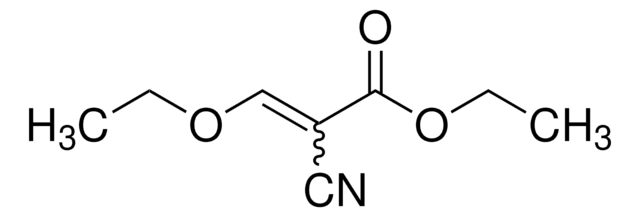

![2-[Bis(methylthio)methylene]malononitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)


![2-[(4-Bromophenyl)methylene]malononitrile](/deepweb/assets/sigmaaldrich/product/structures/581/517/49220e75-b85d-4d94-b647-d741dce149a6/640/49220e75-b85d-4d94-b647-d741dce149a6.png)




