15485
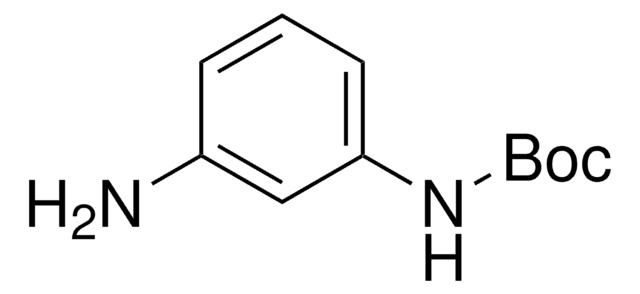
N-Boc-p-phenylenediamine
≥97.0% (NT)
Synonym(s):
4-(tert-Butoxycarbonylamino)aniline, tert-Butyl-4-aminophenylcarbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3COCONHC6H4NH2
CAS Number:
Molecular Weight:
208.26
Beilstein/REAXYS Number:
2969618
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97.0% (NT)
form
powder
reaction suitability
reagent type: cross-linking reagent
functional group
Boc
amine
SMILES string
NC1=CC=C(NC(OC(C)(C)C)=O)C=C1
InChI
1S/C11H16N2O2/c1-11(2,3)15-10(14)13-9-6-4-8(12)5-7-9/h4-7H,12H2,1-3H3,(H,13,14)
InChI key
WIVYTYZCVWHWSH-UHFFFAOYSA-N
Application
N-Boc-p-phenylenediamine can be used as:
- A reactant to prepare perylene monoimide-based dyes for dye-sensitized solar cell applications.
- A starting material to synthesize covalent organic frameworks, which are used as proton exchange membranes for hydrogen fuel cell applications.
- A reactant in the synthesis of bestatin derived hydroxamic acids as potent pan-HDAC inhibitors.
Other Notes
Mono-N-acylated diamine, precursor of different Para-substituted anilines (e.g., 4-azido aniline); Synthesis of photoactivable derivative of ouabaine.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M.P. Goeldner et al.
Trends in Biochemical Sciences, 7, 310-310 (1982)
Synthesis, photophysical and photovoltaic investigations of acceptor-functionalized perylene monoimide dyes for nickel oxide p-type dye-sensitized solar cells
Le Pleux L, et al.
Energy & Environmental Science, 4(6), 2075-2084 (2011)
Combined Intrinsic and Extrinsic Proton Conduction in Robust Covalent Organic Frameworks for Hydrogen Fuel Cell Applications
Yang Y, et al.
Angewandte Chemie (International ed. in English), 59(9), 3678-3684 (2020)
Development of a Bestatin-SAHA Hybrid with Dual Inhibitory Activity against APN and HDAC
Cao J, et al.
Molecules (Basel), 25(21), 4991-4991 (2020)
E. Escher et al.
Helvetica Chimica Acta, 62, 1217-1217 (1979)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![4-[(N-Boc)aminomethyl]aniline 97%](/deepweb/assets/sigmaaldrich/product/structures/341/155/530c425c-7e6e-435e-a28a-9d40b05b938a/640/530c425c-7e6e-435e-a28a-9d40b05b938a.png)








