All Photos(1)
About This Item
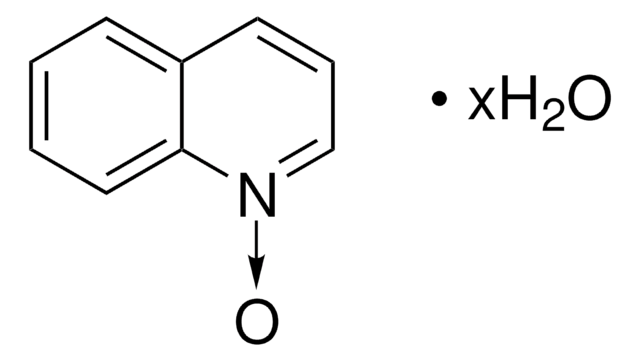
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein/REAXYS Number:
105257
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
solid
bp
270 °C (lit.)
mp
62-67 °C (lit.)
SMILES string
[O-][n+]1ccccc1
InChI
1S/C5H5NO/c7-6-4-2-1-3-5-6/h1-5H
InChI key
ILVXOBCQQYKLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyridine N-oxide axle with [2]rotaxanes was synthesized via an anion templated threading-followed-by-stoppering strategy.
Application
Pyridine N-oxide was used to study the FTIR spectra of pyridine N-oxide in acetonitrile.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
289.4 °F - closed cup
flash_point_c
143 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xue Gong et al.
Organic letters, 13(7), 1766-1769 (2011-03-11)
A Pd(II)-catalyzed oxidative coupling between pyridine N-oxides and N-substituted indoles via 2-fold C-H bond activation was achieved with high selectivity using Ag(2)CO(3) as an oxidant.
Jinshui Chen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(30), 7268-7276 (2009-07-07)
Optically active chiral alkyl chlorides are valuable compounds because of their bioactivity and versatile synthetic utility. Accordingly, the ring opening of epoxides with a chloride nucleophile stands as an important goal in asymmetric catalysis. We describe herein recent advances in
Masahito Murai et al.
Chemical communications (Cambridge, England), 48(61), 7622-7624 (2012-06-26)
Gold(I)-catalysed tandem oxygen-transfer/cycloisomerisation reaction of 2-(2-propynyl)pyridine N-oxides provides an atom-economical route to indolizinone frameworks.
Munawar Hussain et al.
Organic letters, 15(1), 54-57 (2012-12-22)
The synthesis of optically active piperidines by enantioselective addition of aryl Grignard reagents to pyridine N-oxides and lithium binolate followed by reduction is reported for the first time. The reaction results in high yields (51-94%) in combination with good ee
Santiago Barroso et al.
Organic letters, 13(3), 402-405 (2010-12-24)
Enantioselective nitrone cycloadditions with 2-alkenoyl pyridine N-oxides as dipolarophiles have been reported. The reaction is catalyzed by Cu(II)-BOX complexes to give the expected isoxazolidine products with high diastereo- and enantioselectivity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service