All Photos(1)
About This Item
Linear Formula:
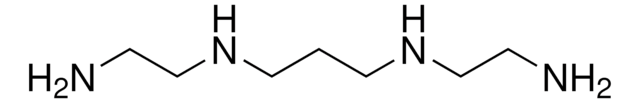
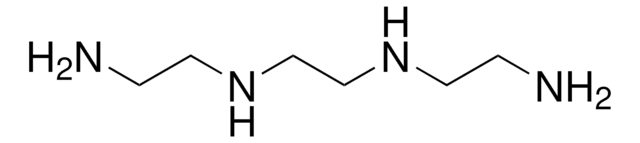
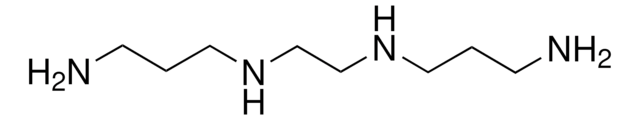
H2N(CH2)3NHCH2CH2NH2
CAS Number:
Molecular Weight:
117.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
liquid
refractive index
n20/D 1.4815 (lit.)
density
0.928 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
NCCCNCCN
InChI
1S/C5H15N3/c6-2-1-4-8-5-3-7/h8H,1-7H2
InChI key
DTSDBGVDESRKKD-UHFFFAOYSA-N
Application
N-(2-Aminoethyl)-1,3-propanediamine has been used as a templating agent in the synthesis of a new open framework iron(III) phosphite {(C5H18N3)[Fe3(HPO3)6].3H2O}. It has also been used in the preparation of dinitrocobalt(III) compound: (11-amino-4-methyl-5,8-diazaundeca-2,4-dien-2-olato-kappa4N(5,8,11),O)-dinitrocobalt(III), [Co(C10H20N3O)(NO2)2].
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1A - Skin Sens. 1
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 2
flash_point_f
206.6 °F - closed cup
flash_point_c
97 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Chun et al.
Acta crystallographica. Section C, Crystal structure communications, 57(Pt 1), 33-35 (2001-02-15)
The reaction of Co(acac)3 with N-(2-aminoethyl)-1,3-propanediamine in the presence of NaNO2 results in the preparation of an unexpected dinitrocobalt(III) compound, (11-amino-4-methyl-5,8-diazaundeca-2,4-dien-2-olato-kappa4N(5,8,11),O)-dinitrocobalt(III), [Co(C10H20N3O)(NO2)2], containing the tetradentate anion of 11-amino-4-methyl-5,8-diazaundeca-2,4-dien-2-ol. Two isomers of the compound were obtained by recrystallization of the crude
U-Chan Chung et al.
Inorganic chemistry, 45(22), 8965-8972 (2006-10-24)
A new open framework iron(III) phosphite with formula (C5H18N3)[Fe3(HPO3)6].3H2O has been prepared by hydrothermal synthesis with N-(2-aminoethyl)-1,3-propanediamine as a templating agent. The crystal structure was solved from single-crystal X-ray diffraction data in the trigonal space group R. The unit cell
Naciye Esma Tirtom et al.
Physical chemistry chemical physics : PCCP, 22(21), 11928-11935 (2020-05-21)
Polyamines are naturally occurring cationic molecules in cells. In addition to their roles in modulating gene expression and cell proliferation, they have been shown to stimulate DNA recombination. The molecular mechanism for stimulation is not clear. We utilized single-molecule tethered
Lucas Vu et al.
Theranostics, 2(12), 1160-1173 (2013-02-06)
A focused library of twenty-one cationic poly(amino ethers) was synthesized following ring-opening polymerization of two diglycidyl ethers by different oligoamines. The polymers were screened in parallel for plasmid DNA (pDNA) delivery, and transgene expression efficacies of individual polymers were compared
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service