123188
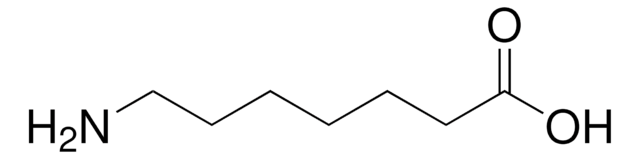
5-Aminovaleric acid
97%, for peptide synthesis
Synonym(s):
5-AVA, 5-Aminopentanoic acid, Homopiperidinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2(CH2)4CO2H
CAS Number:
Molecular Weight:
117.15
Beilstein/REAXYS Number:
906833
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
5-Aminovaleric acid, 97%
Quality Level
assay
97%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
158-161 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCCCCC(O)=O
InChI
1S/C5H11NO2/c6-4-2-1-3-5(7)8/h1-4,6H2,(H,7,8)
InChI key
JJMDCOVWQOJGCB-UHFFFAOYSA-N
Gene Information
human ... SLC15A1(6564)
Looking for similar products? Visit Product Comparison Guide
Application
5-Aminovaleric acid (5-AVA) is used:
- In the preparation of (5-AVA)x(MA)1-xPbI3, a perovskite for fabricating printable mesoscopic perovskite solar cell.
- As a spacer in the synthesis of rhenium and technetium-99m labeled insulin.
- To synthesize dipeptides that self-assemble to form nanotubes in the solid state as well as in solution over a wide range of pH.
- As a starting material in the total synthesis of an alkaloid, lycoposerramine Z.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and characterization of rhenium and technetium-99m labeled insulin.
Sundararajan C, et al.
Journal of Medicinal Chemistry, 53(6), 2612-2621 (2010)
Ariel Kwaka et al.
Molecular pharmacology, 94(5), 1289-1297 (2018-09-09)
Nematodes exhibit a vast array of cys-loop ligand-gated ion channels with unique pharmacologic characteristics. However, many of the structural components that govern the binding of various ligands are unknown. The nematode cys-loop GABA receptor uncoordinated 49 (UNC-49) is an important
A hole-conductor?free, fully printable mesoscopic perovskite solar cell with high stability.
Mei A, et al.
Science, 345(6194), 295-298 (2014)
cis-Decahydroquinolines via asymmetric organocatalysis: Application to the total synthesis of lycoposerramine Z.
Bradshaw B, et al.
Organic Letters, 15(2), 326-329 (2012)
G A van den Berg et al.
Journal of chromatography, 339(2), 223-231 (1985-05-03)
The mass fragmentographic identification of N-(2-carboxyethyl)-4-amino-n-butyric acid, N-(3-aminopropyl)-N1-(2-carboxyethyl)-1,4-diaminobutane, N,N1-bis(2-carboxyethyl)-1,4-diaminobutane, and delta-aminovaleric acid in acid-hydrolysed urines of a normal person and two cancer patients is described. A previous study, in which the metabolic fate of intraperitoneally injected polyamines in rats was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service