119474
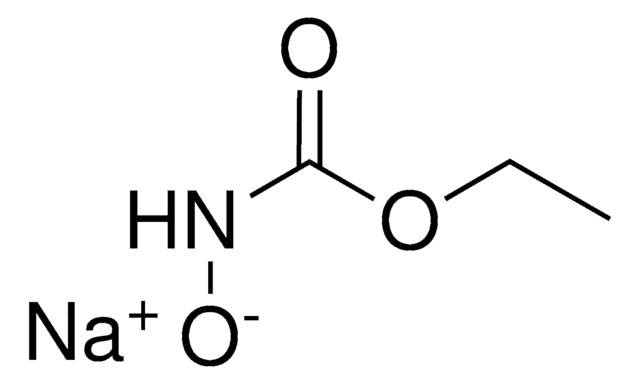
N-Hydroxyurethane
Synonym(s):
N-Carbethoxyhydroxylamine, Ethyl N-hydroxycarbamate, Hydroxycarbamic acid ethyl ester, NSC 71045, NSC 83629
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HONHCOOCH2CH3
CAS Number:
Molecular Weight:
105.09
Beilstein/REAXYS Number:
1747529
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.445 (lit.)
bp
113-116 °C/3 mmHg (lit.)
storage temp.
−20°C
SMILES string
CCOC(=O)NO
InChI
1S/C3H7NO3/c1-2-7-3(5)4-6/h6H,2H2,1H3,(H,4,5)
InChI key
VGEWEGHHYWGXGG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Hydroxyurethane was used to synthesize N-methyl-O-benzylhydroxylamine and N-isopropyl-O-methylhydroxylamine.
Reactant involved in:
- Synthesis of molecules used for intermolecular Sharpless aminohydroxylation reactions
- Intermolecular ortho-C-H amidation of anilides
- Cinchona alkaloid-catalyzed asymmetric cycloaddition
- Allylic arylation
Biochem/physiol Actions
N-Hydroxyurethane causes the chromosomal fragmentation at millimolar concentrations and cell toxicity in cultured normal human leukocytes.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kinetic applications of electron paramagnetic resonance spectroscopy. 28. N-Alkoxy-N-alkylamino, N-alkoxyamino, and N-alkoxyanilino radicals.
Kaba RA and Ingold KU
Journal of the American Chemical Society, 98(23), 7375-7380 (1976)
P M Weiss et al.
Biochemistry, 23(19), 4346-4350 (1984-09-11)
The true substrate for the pyruvate kinase catalyzed phosphorylation of hydroxylamine at high pH which is activated by bicarbonate is shown to be N-hydroxycarbamate, since a lag is seen when the reaction is started by the addition of bicarbonate or
Katsuhisa Sakano et al.
Free radical biology & medicine, 33(5), 703-714 (2002-09-05)
Carcinogenic urethane (ethyl carbamate) forms DNA adduct via epoxide, whereas carcinogenic methyl carbamate can not. To clarify a mechanism independent of DNA adduct formation, we examined DNA damage induced by N-hydroxyurethane, a urethane metabolite, using 32P-5'-end-labeled DNA fragments. N-hydroxyurethane induced
T Nomura et al.
Cancer research, 43(11), 5156-5162 (1983-11-01)
To learn the effects of tumor inhibitors on chemically induced malformations, caffeine, antipain, and 13-trans-retinoic acid were given to pregnant ICR/Jcl mice after a single dose of urethan, N-hydroxyurethan, N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea, or 4-nitroquinoline 1-oxide, which induces about 50% of the
K Sugihara et al.
Journal of pharmacobio-dynamics, 6(9), 677-683 (1983-09-01)
The present study provides the evidence that liver aldehyde oxidase in the presence of its electron donors can catalyze the reduction of N-hydroxyurethane to urethane under anaerobic conditions. Guinea pig liver 9000 X g supernatant and cytosol, but not liver
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service