All Photos(1)
About This Item
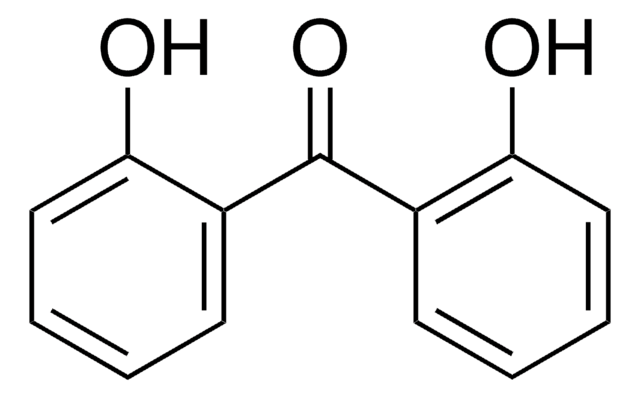
Linear Formula:
HOC6H4COC6H5
CAS Number:
Molecular Weight:
198.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
bp
171-173 °C/12 mmHg (lit.)
mp
37-39 °C (lit.)
SMILES string
Oc1ccccc1C(=O)c2ccccc2
InChI
1S/C13H10O2/c14-12-9-5-4-8-11(12)13(15)10-6-2-1-3-7-10/h1-9,14H
InChI key
HJIAMFHSAAEUKR-UHFFFAOYSA-N
Related Categories
General description
- 2-Hydroxybenzophenone forms copolymer 2-hydroxy, 3-allyl, 4,4′-dimethoxybenzophenone with methyl methacrylate.
- It forms photostabilizer compounds with 2,2,6,6-tetramethylpiperidine under phase transfer catalysis conditions.
- It reacts with 3-aminophenyl boronic acid to form macrocyclic boronates.
Application
2-Hydroxybenzophenone was used to quantify the metabolites of unmethylated extracts from the supernatants of Pseudomonas acidovorans cultures grown on 1,1-diphenylethylene.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vanessa Giannetti et al.
Journal of the science of food and agriculture, 97(7), 2191-2198 (2016-11-02)
The lack of harmonized European legislation on food packaging led the Confederation of European Paper Industries to the proposal of a voluntary Industry Guideline for the compliance of paper and board materials for food contact applications. In the present work
Transient absorption spectra of 2-hydroxybenzophenone photostabilizers
Merritt C, et al.
Chemical Physics Letters, 69(1), 169-173 (1980)
Synthesis and application of new combined 2, 2, 6, 6-tetramethylpiperidine-2-hydroxybenzophenone 1, 3, 5-triazine derivatives as photostabilizers for polymer materials
Bojinov V and Grabchev I
J. Photochem. Photobiol., A, 146(3), 199-205 (2002)
New boron macrocycles based on self-assembly of Schiff bases
Barba V, et al.
Journal of Organometallic Chemistry, 622(1), 259-264 (2001)
A G Hay et al.
Applied and environmental microbiology, 64(6), 2141-2146 (1998-06-03)
1,1-Dichloro-2,2-bis(4-chlorophenyl)ethylene (DDE), a toxic breakdown product of 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT), has traditionally been viewed as a dead-end metabolite: there are no published reports detailing enzymatic ring fission of DDE by bacteria in either soil or pure culture. In this study, we
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service