All Photos(1)
About This Item
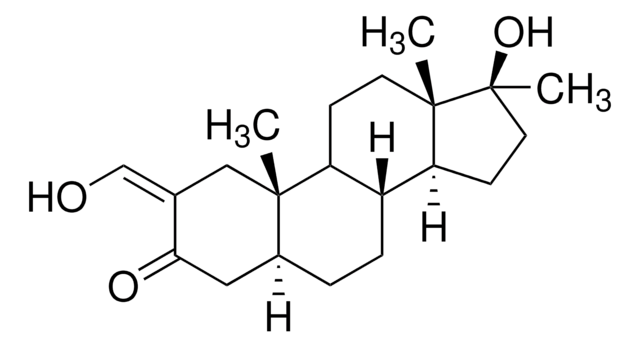
Empirical Formula (Hill Notation):
C4H8O4
CAS Number:
Molecular Weight:
120.10
Beilstein/REAXYS Number:
1721696
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
Recommended Products
form
syrup
Quality Level
optical activity
[α]20/D -16.4 to -9.8 °, c = 2% (w/v) in water
concentration
≥60%
storage temp.
2-8°C
SMILES string
O[C@H]1COC(O)[C@@H]1O
InChI
1S/C4H8O4/c5-2-1-8-4(7)3(2)6/h2-7H,1H2/t2-,3+,4?/m0/s1
InChI key
FMAORJIQYMIRHF-URORKIPUSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Benjamin D Heuberger et al.
Organic letters, 8(25), 5809-5811 (2006-12-01)
Cytosine TNA promotes nonenzymatic, template-directed oligomerization of complementary activated rGMP, leading to selective and efficient formation of RNA products. This process models "genetic takeover" of a pre-RNA by RNA. [reaction: see text]
Zhenyu Dai et al.
Archives of biochemistry and biophysics, 463(1), 78-88 (2007-05-01)
We isolated a novel acid-labile yellow chromophore from the incubation of lysine, histidine and d-threose and identified its chemical structure by one and two-dimensional NMR spectroscopy combined with LC-tandem mass spectrometry. This new cross-link exhibits a UV absorbance maximum at
María Ruiz et al.
The Journal of organic chemistry, 73(6), 2240-2255 (2008-02-28)
A general strategy for the synthesis of 1-deoxy-azasugars from a chiral glycine equivalent and 4-carbon building blocks is described. Diastereoselective aldol additions of metalated bislactim ethers to matched and mismatched erythrose or threose acetonides and intramolecular N-alkylation (by reductive amination
Veerle Kempeneers et al.
Chemistry & biodiversity, 1(1), 112-123 (2006-12-29)
TNA (alpha-L-threose nucleic acids) is potentially a natural nucleic acid, that might have acted as an evolutionary alternative of RNA. We determined the catalytic activity of hammerhead ribozymes containing a threofuranosyl-modified nucleoside at position U4 and U7, and compared these
Jussi Kinnunen et al.
Journal of biomedical optics, 17(9), 97003-97003 (2012-09-15)
Extensive collagen cross-linking affects the mechanical competence of articular cartilage: it can make the cartilage stiffer and more brittle. The concentrations of the best known cross-links, pyridinoline and pentosidine, can be accurately determined by destructive high-performance liquid chromatography (HPLC). We
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service