SRP6133
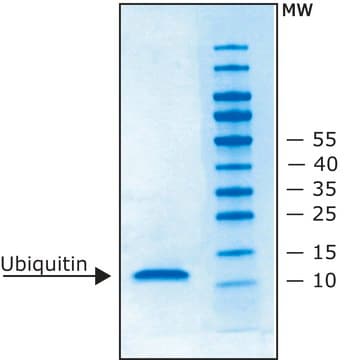
Ubiquitin-K48 human
recombinant, expressed in E. coli, ≥85% (SDS-PAGE)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
biological source
human
recombinant
expressed in E. coli
assay
≥85% (SDS-PAGE)
form
liquid
mol wt
8.732 kDa
packaging
pkg of 500 μg
NCBI accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... RPS27A(6233)
General description
Ubiquitin is a small polypeptide that can be conjugated via its C-terminus to amine groups of lysine residue on target proteins. This conjunction is referred to as monoubiquitylation. Additional ubiquitin moieties can be subsequently conjugated to this initial ubiquitin, utilizing any one of the seven lysine residues on the surface of ubiquitin. The formation of these ubiquitin chains is referred to as polyubiquitylation. This tag-free recombinant form of human ubiquitin is engineered to have all available lysines mutated to arginines, except at position 48. This molecule, therefore, can only form polyubiquitin chains of K48 linkage type. Covalent attachment of ubiquitin to other proteins serves various functions, but its major role is to target cellular proteins for destruction. Cellular components that activate, transfer, remove, or simply recognize ubiquitin number in the hundreds, perhaps even in the thousands. In light of this complexity the ubiquitin pathway is ideal for a systems biology approach. Ubiquitin plays a very important role in regulated non-lysosomal ATP dependent protein degradation. The Ub-proteasome proteolytic pathway, which is a complex process, is implicated to be of great importance for regulating numerous cellular processes.
Physical form
4 mg/mL in 50 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol.
Preparation Note
Centrifuge the vial prior to opening.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service