S8065
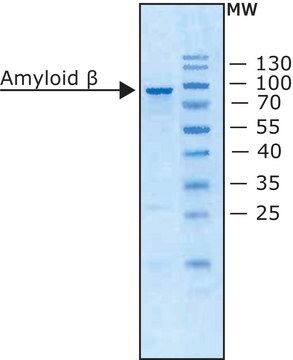
Amyloid Precursor Protein α (304-612), Secreted human
>90% (SDS-PAGE), recombinant, expressed in E. coli (N-terminal histidine tagged), buffered aqueous solution
Synonym(s):
sAPPα (304-612)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Biochem/physiol Actions
Secreted Amyloid Precursor Protein α (sAPPα) is a 612 amino acid protein produced from the ubiquitously expressed β-Amyloid precursor protein (βAPP), 695 isoform, by α-secretase cleavage. sAPPα (304-612) is a fragment of sAPPα designed to lack the N-terminal domain of the full length protein.
Physical form
0.2 μm filtered solution in phosphate buffered saline, pH 7.4.
Preparation Note
Expressed as a soluble protein and purified under non-denaturing conditions.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mattson, M.P.
The Journal of Neuroscience, 25, 439-439 (1994)
Greenfield, J.P.,
Frontiers in Bioscience, 5, 72-72 (2000)
S W Barger et al.
Nature, 388(6645), 878-881 (1997-08-28)
A role for beta-amyloid precursor protein (beta-APP) in the development of Alzheimer's disease has been indicated by genetics, and many conditions in which beta-APP is raised have been associated with an increased risk of Alzheimer's disease or an Alzheimer's-like pathology.
L W Jin et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 14(9), 5461-5470 (1994-09-01)
Amyloid beta/A4 protein precursor (APP) is secreted into medium by most cultured cells and can function as an autocrine factor. To study the biological function of secreted forms of APP (sAPP) on neurons, we used a clonal CNS neuronal line
D H Small et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 14(4), 2117-2127 (1994-04-01)
The amyloid protein precursor (APP) of Alzheimer's disease is synthesized as an integral transmembrane protein that is released from cells in culture following proteolytic cleavage. The function of released APP is not known, although there is evidence that the protein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service