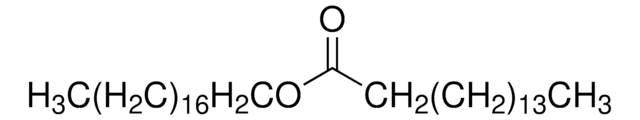
O3380
Oleyl oleate
≥99%
Synonym(s):
Oleyl oleate, Oleic acid oleyl ester
About This Item
Recommended Products
assay
≥99%
form
liquid
functional group
ester
oleic acid
lipid type
unsaturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCCCCC\C=C/CCCCCCCCOC(=O)CCCCCCC\C=C/CCCCCCCC
InChI
1S/C36H68O2/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-36(37)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h17-20H,3-16,21-35H2,1-2H3/b19-17-,20-18-
InChI key
BARWIPMJPCRCTP-CLFAGFIQSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Group 13 Lewis acid catalyzed synthesis of metal oxide nanocrystals via hydroxide transmetallation.: This research utilizes oleyl oleate as a solvent to facilitate the synthesis of metal oxide nanocrystals, showcasing its effectiveness in nanoparticle production and potential applications in various electronic and optical devices (Gibson et al., 2021).
- High-level accumulation of oleyl oleate in plant seed oil by abundant supply of oleic acid substrates to efficient wax ester synthesis enzymes.: This article details the genetic engineering approaches to enhance oleyl oleate content in plant oils, aiming at industrial applications such as biofuels and biolubricants (Yu et al., 2018).
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
452.3 °F
flash_point_c
233.5 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service