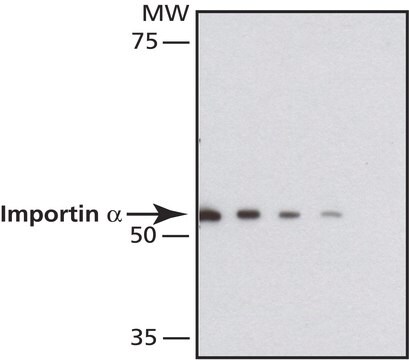
I9781
Importin β1 human
≥80% (SDS-PAGE), recombinant, expressed in E. coli (N-terminal histidine tagged), buffered aqueous glycerol solution
Synonym(s):
Importin β, Karyopherin β1, p97
About This Item
Recommended Products
recombinant
expressed in E. coli (N-terminal histidine tagged)
assay
≥80% (SDS-PAGE)
form
buffered aqueous glycerol solution
mol wt
~97 kDa by SDS-PAGE
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... KPNB1(3837)
General description
Biochem/physiol Actions
The mechanism of importin β action in nuclear import can be demonstrated by the well-studied import of proteins containing classical NLS. Importin β forms a complex with Importin α, which, in turn, binds the cargo protein via its NLS. The Impβ/Impα/cargo complex translocates into the nucleus. When the complex reaches the nuclear site of the NPC, Ran-GTP binds the Impβ to form Impβ/Ran-GTP complex and released the Impα and the cargo protein. The Impβ/Ran-GTP complex is then exported to the cytoplasm where the complex dissociates upon hydrolysis of GTP to GDP, making Impβ ready for a new import cycle.
Physical form
Storage Class
12 - Non Combustible Liquids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service