All Photos(1)
About This Item
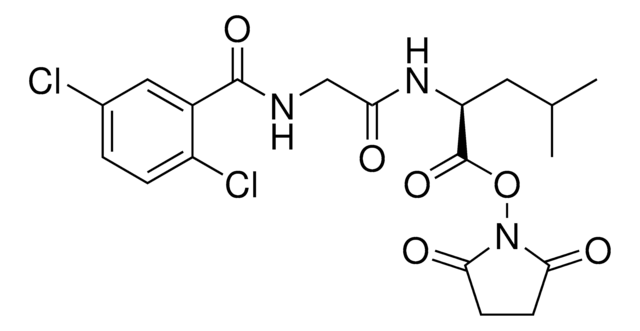
Empirical Formula (Hill Notation):
C18H34N4O5
CAS Number:
Molecular Weight:
386.49
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
assay
≥97% (HPLC)
storage temp.
−20°C
SMILES string
CC(C)CC(NC(=O)C(C)NC(=O)C(CC(C)C)NC(=O)C(C)N)C(O)=O
InChI
1S/C18H34N4O5/c1-9(2)7-13(21-15(23)11(5)19)17(25)20-12(6)16(24)22-14(18(26)27)8-10(3)4/h9-14H,7-8,19H2,1-6H3,(H,20,25)(H,21,23)(H,22,24)(H,26,27)
InChI key
KQRHTCDQWJLLME-UHFFFAOYSA-N
Amino Acid Sequence
Ala-Leu-Ala-Leu
Biochem/physiol Actions
A peptidase labile sequence used to link drugs to proteins or lipophilic carriers.
Ala-Leu-Ala-Leu (ALAL), a peptidase labile (cathepsin B) prodrug linker, is used to link bioavailable drugs to protein carriers such as albumin.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Trouet, A., et al. et al.
Targeting of Drugs, 19-30 (1982)
Björn Schmid et al.
Bioconjugate chemistry, 18(3), 702-716 (2007-03-24)
We have recently validated a macromolecular prodrug strategy for improved cancer chemotherapy based on two features: (a) rapid and selective binding of thiol-reactive prodrugs to the cysteine-34 position of endogenous albumin and (b) acid-sensitive promoted or enzymatic release of the
M Studer et al.
Bioconjugate chemistry, 3(5), 420-423 (1992-09-01)
The synthesis of a protected bifunctional analog of ethylenediaminetetraacetic acid (EDTA) is described. The molecule contains an aminobenzyl moiety that allows the easy attachment of the chelating agent to a wide variety of groups. Examples of reaction with the C-termini
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service