A0796
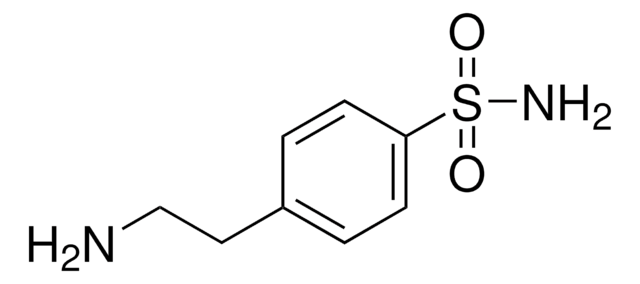
p-Aminomethylbenzenesulfonamide–Agarose
saline suspension
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
23151817
Recommended Products
form
saline suspension
extent of labeling
≥6 mg per mL
matrix
crosslinked 4% beaded agarose
matrix activation
cyanogen bromide
matrix attachment
amino
matrix spacer
1 atom
storage temp.
2-8°C
Application
p-Aminomethylbenzenesulfonamide is an agarose in saline suspension that can be used in affinity chromatography, protein chromatography and specialty resins. p-Aminomethylbenzenesulfonamide has been used in studies assessing inhibition of mitochondrial carbonic anhydrase and ureagenesis.
Physical form
Suspension in 0.5 M NaCl containing preservative.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B Ulmasov et al.
Proceedings of the National Academy of Sciences of the United States of America, 97(26), 14212-14217 (2000-12-20)
Carbonic anhydrase XII (CA XII) is a transmembrane glycoprotein with an active extracellular CA domain that is overexpressed on cell surfaces of certain cancers. Its expression has been linked to tumor invasiveness. To characterize its catalytic properties, we purified recombinant
S J Dodgson et al.
Journal of applied physiology (Bethesda, Md. : 1985), 60(2), 646-652 (1986-02-01)
The amount of urea produced in 60 min, [urea]t = 60, from intact guinea pig hepatocytes incubated in NH4Cl, oleate, lactate, NaHCO3, and ornithine at 37 degrees C at pH 7.1 is decreased by ethoxzolamide (EZ): Ki,EZ [urea]t = 60
D K Srivastava et al.
Journal of the American Chemical Society, 129(17), 5528-5537 (2007-04-05)
Despite the similarity in the active site pockets of carbonic anhydrase (CA) isozymes I and II, the binding affinities of benzenesulfonamide inhibitors are invariably higher with CA II as compared to CA I. To explore the structural basis of this
B E Alber et al.
Proceedings of the National Academy of Sciences of the United States of America, 91(15), 6909-6913 (1994-07-19)
Carbonic anhydrase (CA) from acetate-grown Methanosarcina thermophila was purified > 10,000-fold (22% recovery) to apparent homogeneity with a specific activity of 4872 units/mg. The estimated native molecular mass of the enzyme is 84 kDa based on gel filtration chromatography. SDS/PAGE
S J Dodgson
Journal of applied physiology (Bethesda, Md. : 1985), 63(5), 2134-2141 (1987-11-01)
The amount of urea synthesized in intact guinea pig hepatocytes in 60 min ([urea]t=60), was determined at 37 degrees C in Krebs-Henseleit buffer plus (in mM) 10 NH4Cl, 5 lactate, and 10 ornithine in 5% CO2-95% O2. The concentrations of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service