04473
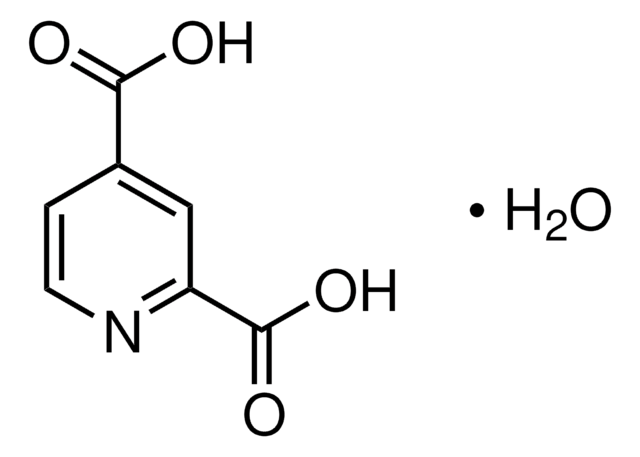
2,4-Pyridinedicarboxylic acid
≥98.0%
Synonym(s):
Lutidinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5NO4
CAS Number:
Molecular Weight:
167.12
Beilstein/REAXYS Number:
131631
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
assay
≥98.0%
98.0-102.0% (T)
SMILES string
OC(=O)c1ccnc(c1)C(O)=O
InChI
1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
InChI key
MJIVRKPEXXHNJT-UHFFFAOYSA-N
Application
2,4-Pyridinedicarboxylic acid is an in vitro and in cell inhibitor, as well as a known inhibitor of the histone lysine demethylases. 2,4-Pyridinedicarboxylic acid has been used in a study to determine that ruthenium(II) complexes exert antimetastatic effects on several tumor cell lines in vitro, achieved mostly by the effect on cell adhesion, migration and angiogenesis. . 2,4-Pyridinedicarboxylic acid has been used in a study to develop an assay that represents the first report of a RapidFire mass spectrometery assay for an epigenetics target.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guo-liang Gu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(1), 209-214 (2008-02-19)
Two novel ligands containing two pyridine-2,6-dicarboxylic acid conjugative units, 4-(2-(2,6-dicarbox-ypyridin-4-yl)vinyl)pyridine-2,6-dicarboxylic acid (L1) and 4-(4-(2-(2,6-dicarboxypyridin-4-yl)vinyl)styryl)pyridine-2,6-dicarboxylic acid (L2) and their complexes with Tb(III) have been synthesized and characterized by elemental analysis, IR spectra and NMR. The ligand synthetic route was optimized and
H M Rowe et al.
Applied spectroscopy, 57(5), 532-537 (2003-12-09)
An adaptation of square-wave gated phase-modulation (GPM) fluorimetry allows for self-referenced intensity measurements without the complexity of dual excitation or dual emission wavelengths. This AC technique utilizes square-wave excitation, gated detection, a reference emitter, and a sensor molecule. The theory
Michael Raghunath et al.
Biochemical and biophysical research communications, 378(4), 766-771 (2008-12-11)
The rapid vascularisation of biomaterials and engineered tissue after implantation is a current unmet need. To this end, we explored the pharmacological option of inducing neovascularisation using compounds that inhibit hypoxia-induced factor-1alpha prolyl hydroxylase. This stabilises hypoxia inducible factor-1alpha and
John R G Sander et al.
Journal of pharmaceutical sciences, 99(9), 3676-3683 (2010-06-25)
We report on a co-crystal of acetaminophen (APAP) and 2,4-pyridinedicarboxylic acid (PDA). The co-crystal was discovered by screening using the solution-mediated phase transformation (SMPT) technique. Despite the bulk solids of each component being white in color, the new co-crystal phase
C K Derian et al.
The Journal of biological chemistry, 264(12), 6615-6618 (1989-04-25)
While a role has been ascribed to the gamma-carboxyglutamate (Gla) residues in vitamin K-dependent coagulation proteins and the enzyme catalyzing this posttranslational modification has been identified and partially characterized, both the functional significance of a second posttranslationally synthesized amino acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service