89136
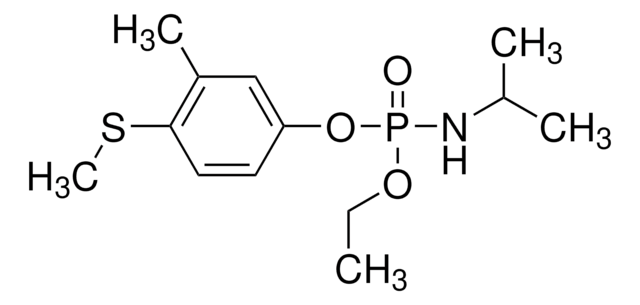
Fenamiphos-(S-methyl-d3)
PESTANAL®, analytical standard
Synonym(s):
Fenamiphos-d3, Phenamiphos-d3
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13D3H19NO3PS
Molecular Weight:
306.38
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
assay
≥98.0% (GC)
shelf life
limited shelf life, expiry date on the label
application(s)
agriculture
format
neat
storage temp.
2-8°C
SMILES string
O=P(NC(C)C)(OC1=CC(C)=C(SC([2H])([2H])[2H])C=C1)OCC
General description
Fenamiphos-(S-methyl-d3) is a deuterated isotope analog of fenamiphos-(S-methyl), a derivative of the broad-spectrum, non-volatile organophosphorus nematicide fenamiphos, wherein S-methyl protons are replaced by deuterium.
Application
Isotope-labeled compounds are increasingly used in isotope dilution mass spectrometry (IDMS) for the quantitative analysis of pesticides. The major advantage of using this technique is that the isotope-labeled compounds have nearly the same physical properties as their non-labeled counterpart analogs, and thus show identical behavior during the workup and sample preparation process. This helps in overcoming the problems of matrix effects generally encountered in the usual LC-MS/GC-MS analysis potentially resulting in biased results. By spiking the sample before workup with its isotope labeled analog, the loss of analyte that leads to matrix effects can be determined and compensated.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
signalword
Danger
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bioremedial potential of fenamiphos and chlorpyrifos degrading isolates: influence of different environmental conditions
Singh BK, et al.
Soil Biology and Biochemistry, 38(9), 2682-2693 (2006)
Land use effects on sorption of pesticides and their metabolites in sandy soils. I. Fenamiphos and two metabolites, fenamiphos sulfoxide and fenamiphos sulfone, and fenarimol and azinphos methyl
Oliver DP, et al.
Australian Journal of Soil Research, 41(5), 847-860 (2003)
Tanya P Cáceres et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 43(7), 605-610 (2008-09-23)
Fenamiphos (0-ethyl-0(3-methyl-4-methylthiophenyl)-isopropylamido-phosphate) is a widely used nematicide and insecticide in bowling greens and agriculture, but information on its sorption including its metabolites is limited. Hence, the sorption of fenamiphos (nematicide) and its major degradation products fenamiphos sulfoxide (FSO) and fenamiphos
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service