74011
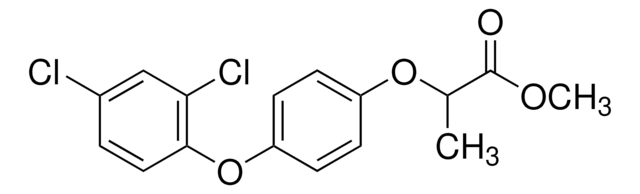
(±)-Diclofop
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Synonym(s):
(RS)-2-[4-(2,4-Dichlorophenoxy)phenoxy]propionic acid
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
format
neat
storage temp.
2-8°C
SMILES string
CC(Oc1ccc(Oc2ccc(Cl)cc2Cl)cc1)C(O)=O
InChI
1S/C15H12Cl2O4/c1-9(15(18)19)20-11-3-5-12(6-4-11)21-14-7-2-10(16)8-13(14)17/h2-9H,1H3,(H,18,19)
InChI key
OOLBCHYXZDXLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com
(+/–)-Diclofop is a chiral herbicide that belongs to the chemical class of chiral aryloxyphenoxypropionate compounds. The actual herbicidal active ingredient― carboxylic acid, is released after application through hydrolysis of the ester. It is absorbed mainly through leaves and inhibits the biosynthesis of fatty acids by suppressing the activity of acetyl CoA carboxylase (ACCase). It is used for the post-emergence control of wild oats, wild millets, and other annual grass weeds commonly occurring in wheat, barley, rye, red fescue, and broad-leaved crops.
It was included on 1st June 2011 in Annex I of Directive 91/414/EEC by the European Commission Directive 2011/45/EU. It is authorized for use under EC Regulation No 1107/2009, as per the Commission Implementing Regulation (EU) No 540/2011, however it is a candidate for substitution.
Application
The (+/–)-Diclofop CRM can also be used as following:
- To evaluate the likely enantioselective oxidative stress produced in Microcystis aeruginosa by diclofop acid
- For analyzing the phytotoxic effects of diclofop acid enantiomers on the plant Arabidopsis thaliana
- To study the enantioselective toxicity of diclofop acid on the non-target rice Xiushui 63 seedlings
- In the chiral separation of diclofop-acid using one- and two- dimensional HPLC methods
- To determine the enantioselective toxicity and degradation of diclofop in three algal cultures
Recommended products
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service