64120
Methacryloyl chloride
purum, dist., ≥97.0% (GC), contains ~0.02% 2,6-di-tert-butyl-4-methylphenol as stabilizer
Synonym(s):
2-Methylprop-2-enoyl chloride, Methacrylic acid chloride, Methacrylyl chloride
About This Item
Recommended Products
grade
purum
Quality Level
assay
≥97.0% (GC)
quality
dist.
contains
~0.02% 2,6-di-tert-butyl-4-methylphenol as stabilizer
impurities
≤1000 mg/kg total sulfur (as SO4)
refractive index
n20/D 1.442 (lit.)
n20/D 1.444
bp
95-96 °C (lit.)
density
1.08 g/mL at 20 °C
1.07 g/mL at 25 °C (lit.)
storage temp.
2-8°C
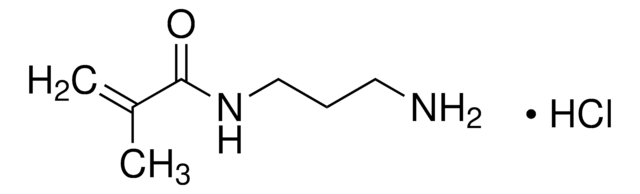
SMILES string
CC(=C)C(Cl)=O
InChI
1S/C4H5ClO/c1-3(2)4(5)6/h1H2,2H3
InChI key
VHRYZQNGTZXDNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A monomer in the synthesis of amphiphilic fluorescent copolymers via reversible addition-fragmentation chain transfer (RAFT) polymerization. The resulting copolymers have smart pH sensitivity and fluorescence properties, which make them suitable for use in cell imaging.
- A monomer in the emulsion polymerization process to prepare a fluorescence probe, which is used for the detection of metronidazole.
- A surface modifier for microcrystalline cellulose (MCC) to improve its compatibility with low-density polyethylene (LDPE) in the preparation of LDPE-based composites.
- A key component in the synthesis of carboxymethyl cellulose acrylates, which find applications in the development of pH-sensitive hydrogels.
- A monomer in the synthesis of methacrylate-based hydrogel materials, which are widely used in the production of soft contact lenses.
- To prepare methacryloyl-functional benzoxazine monomer.
- As a precursor to prepare N-methacryloyl-(L)-histidinemethylester(MAH), a metal chelating monomer.
replaced by
signalword
Danger
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
55.0 °F - closed cup
flash_point_c
12.8 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[2-(Acryloyloxy)ethyl]trimethylammonium chloride solution 80 wt. % in H2O, contains 600 ppm monomethyl ether hydroquinone as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/393/326/f7e19585-5431-4220-81b5-f458de6d63d0/640/f7e19585-5431-4220-81b5-f458de6d63d0.png)
![[2-(Methacryloyloxy)ethyl]trimethylammonium chloride solution 75 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/316/612/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe/640/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe.png)