57619
Acetic acid – triethylamine solution 1:1
suitable for HPLC, 2M:2M aqueous solution, LiChropur™
Synonym(s):
Triethylamine : Acetic acid 1:1 solution, Triethylammonium acetate solution
About This Item
Recommended Products
form
liquid
technique(s)
HPLC: suitable
λ
neat
UV absorption
λ: 250 nm Amax: ≤0.1
λ: 260 nm Amax: ≤0.05
λ: 300 nm Amax: ≤0.01
λ: 500 nm Amax: ≤0.01
SMILES string
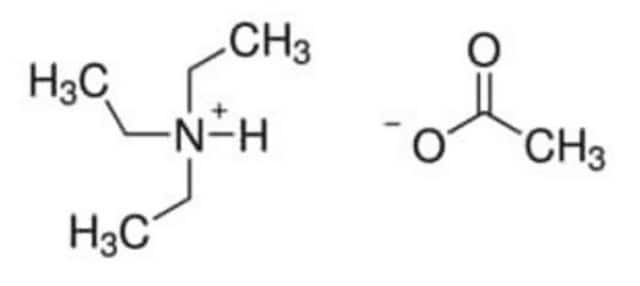
CC(O)=O.CCN(CC)CC
InChI
1S/C6H15N.C2H4O2/c1-4-7(5-2)6-3;1-2(3)4/h4-6H2,1-3H3;1H3,(H,3,4)
InChI key
AVBGNFCMKJOFIN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Direct enantiomeric resolution of betaxolol with application to analysis of pharmaceutical products.: This study presents a method for the direct enantiomeric resolution of betaxolol, where acetic acid and triethylamine are used to facilitate the chiral separation in analytical processes, showcasing its application in pharmaceutical analysis (Hefnawy et al., 2007).
Legal Information
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service