46074
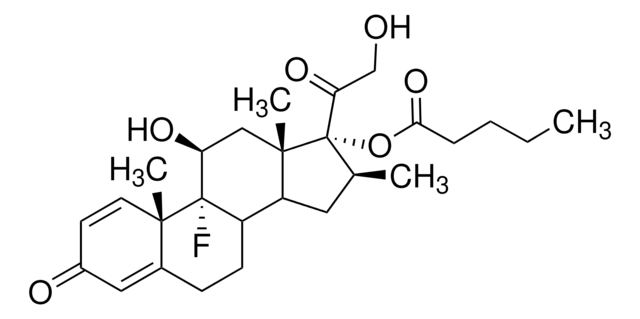
Betamethasone 17-valerate
VETRANAL®, analytical standard
Synonym(s):
1,4-Pregnadiene-11β,17α,21-triol-9α-fluoro-16β-methyl-3,20-dione 17-valerate, 9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione 17-valerate, 9α-Fluoro-16β-methylprednisolone 17-valerate, Betnovate
Select a Size
About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
VETRANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
clinical
format
neat
storage temp.
2-8°C
SMILES string
CCCCC(=O)O[C@@]1([C@@H](C)CC2C3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)CO
InChI
1S/C27H37FO6/c1-5-6-7-23(33)34-27(22(32)15-29)16(2)12-20-19-9-8-17-13-18(30)10-11-24(17,3)26(19,28)21(31)14-25(20,27)4/h10-11,13,16,19-21,29,31H,5-9,12,14-15H2,1-4H3/t16-,19?,20?,21-,24-,25-,26-,27-/m0/s1
InChI key
SNHRLVCMMWUAJD-QDHNOTTGSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B - STOT RE 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service