All Photos(1)
About This Item
Empirical Formula (Hill Notation):
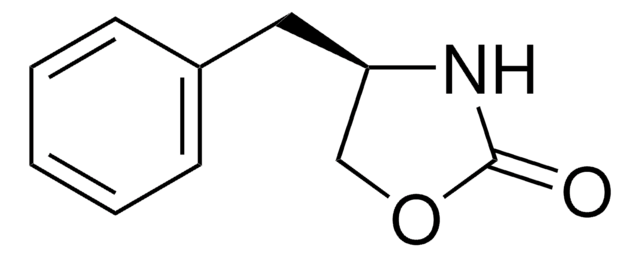
C10H11NOS
CAS Number:
Molecular Weight:
193.27
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97.0%
optical purity
enantiomeric excess: ≥98.0% (HPLC)
mp
63-67 °C
SMILES string
S=C1N[C@H](CO1)Cc2ccccc2
InChI
1S/C10H11NOS/c13-10-11-9(7-12-10)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,11,13)/t9-/m0/s1
InChI key
WJSUXYCBZFLXIK-VIFPVBQESA-N
Application
A highly selective and efficient chiral auxiliary which can be directly reduced to its corresponding aldehyde and the chiral auxiliary by reductive cleavage with diisobutylaluminum hydride.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Velazquez. F.; Olivo, H. F.
Current Organic Chemistry, 6, 303-303 (2002)
M T Crimmins et al.
Organic letters, 2(6), 775-777 (2001-02-07)
[formula: see text] Asymmetric aldol additions using chlorotitanium enolates of thiazolidinethione propionates proceed with high diastereoselectivity for the "Evans" or "non-Evans" syn product depending on the nature and amount of the base used. With (-)-sparteine as the base, selectivities of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![(4S)-(+)-Phenyl-α-[(4S)-phenyloxazolidin-2-ylidene]-2-oxazoline-2-acetonitrile 97%](/deepweb/assets/sigmaaldrich/product/structures/117/759/d4e6e882-8577-4dcf-84fe-506633ae811a/640/d4e6e882-8577-4dcf-84fe-506633ae811a.png)