06670
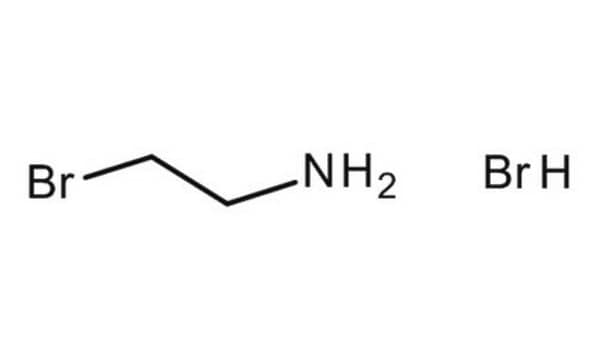
2-Bromoethylamine hydrobromide
purum, ≥97.0% (AT)
Synonym(s):
2-Aminoethyl bromide hydrobromide
Select a Size
About This Item
Recommended Products
grade
purum
Quality Level
assay
≥97.0% (AT)
form
crystalline
bp
181.3 °C/979.2 hPa
mp
170-175 °C (lit.)
170-175 °C
solubility
water: soluble 84.02 g/L at 28 °C
soluble
density
0.98 g/cm3 at 28.3 °C
functional group
amine
bromo
SMILES string
Br.NCCBr
InChI
1S/C2H6BrN.BrH/c3-1-2-4;/h1-2,4H2;1H
InChI key
WJAXXWSZNSFVNG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Amino-functionalized ionic liquid, 1-aminoethyl-3-methylimidazolium hexafluorophosphate ([2-aemim][PF6]). [2-aemim][PF6] is employed as a catalyst in the synthesis of 4H-pyrans derivatives by treating with aromatic aldehydes, malononitrile, ethyl acetoacetate via Knoevenagel condensation reaction.[1]
- 2-(N-aryl-N-aroyl)amino-4,5-dihydrothiazole derivatives via cyclocondensation reaction.[2]
It can be also employed as an alkylating agent for the surface modification of nylon to obtain primary/secondary/tertiary amine groups.[3]
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
142.9 °F - Pensky-Martens closed cup
flash_point_c
61.6 °C - Pensky-Martens closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service