1.46236
Sabouraud Dextrose Agar
ReadyPlate™, ISO, FDA-BAM, EP, USP, plate diam. 90 mm, box of 20 or 120 plates
Synonym(s):
ReadyPlate SDA, SDA
About This Item
Recommended Products
agency
EP
FDA-BAM
ISO
USP
Quality Level
form
plate (ready-to-use)
feature
ready-to-use
manufacturer/tradename
ReadyPlate™
packaging
box of 20 or 120 plates
plate diam.
90 mm
color
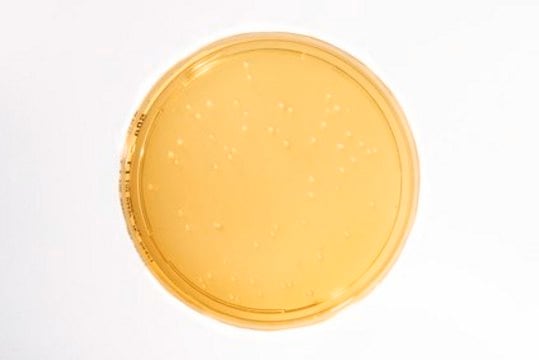
clear yellow Media
pH
5.6 ( in H2O)
application(s)
cosmetics
environmental
food and beverages
microbiology
pharmaceutical
storage temp.
15-25°C
suitability
molds
yeasts
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Media fill tests for beverages ensure that the filling line is not contaminated with spoilage bacteria, yeasts, or molds, during the production of low-acid aseptic beverages.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service