W259519
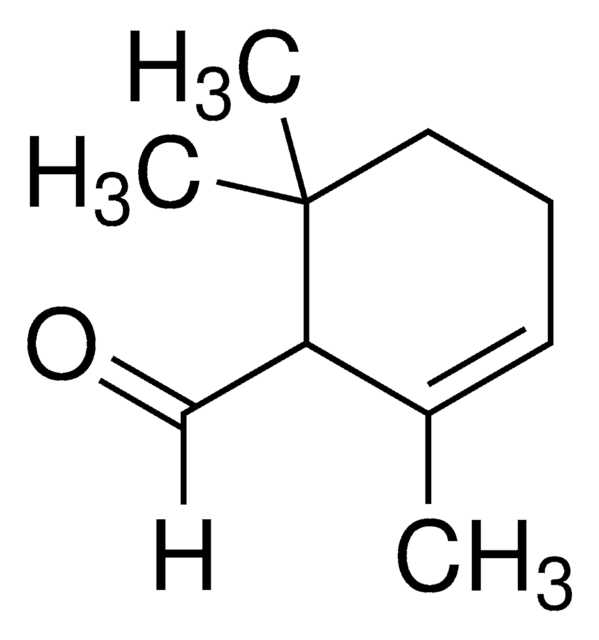
β-Ionone
natural, ≥85%, FG
Synonym(s):
4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one
About This Item
Recommended Products
grade
FG
Halal
Kosher
natural
reg. compliance
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
assay
≥85%
refractive index
n20/D 1.52 (lit.)
bp
126-128 °C/12 mmHg (lit.)
density
0.945 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
SMILES string
CC(=O)\C=C\C1=C(C)CCCC1(C)C
InChI
1S/C13H20O/c1-10-6-5-9-13(3,4)12(10)8-7-11(2)14/h7-8H,5-6,9H2,1-4H3/b8-7+
InChI key
PSQYTAPXSHCGMF-BQYQJAHWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Biochem/physiol Actions
Other Notes
Subscribe to our Newsletter to keep up to date on our latest Flavors and Fragrances offerings.
related product
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 2 - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service