ALD00454
2-(3,3-Diethyl-1-triazenyl)-4-methylbenzenesulfonyl chloride
Synonym(s):
2-[(1E)-3,3-diethyl-1-triazen-1-yl]-4-methyl-benzenesulfonyl chloride
About This Item
Recommended Products
assay
97%
Quality Level
form
powder
availability
available only in USA
mp
76 °C
SMILES string
CCN(CC)\N=N\c1cc(C)ccc1S(Cl)(=O)=O
InChI
1S/C11H16ClN3O2S/c1-4-15(5-2)14-13-10-8-9(3)6-7-11(10)18(12,16)17/h6-8H,4-5H2,1-3H3/b14-13+
InChI key
PDGZSZLMBJSDQC-BUHFOSPRSA-N
Looking for similar products? Visit Product Comparison Guide
application
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Self-react. C
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Baran Group works with Sigma-Aldrich in providing a portfolio of zinc-based reagents promoting difluoromethylation, trifluoromethylation, trifluoroethylation and isopropylation of aryl and heteroaryl motifs. Baran’s lab has also helped introduce a portable desaturase (Tz0Cl), which promotes the installation of alcohol and amine groups and leaves behind a highly useful tosyl group for further transformations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

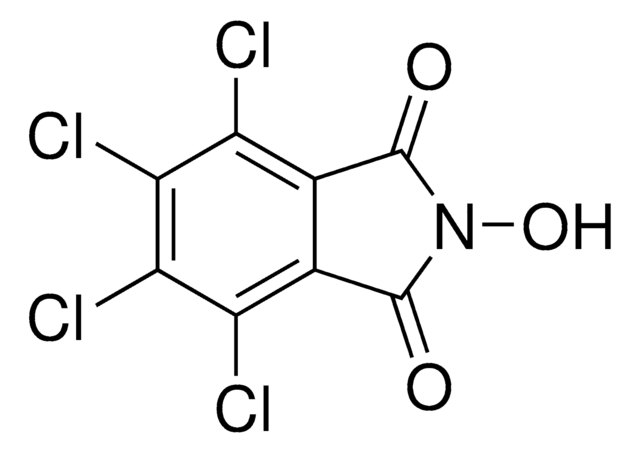
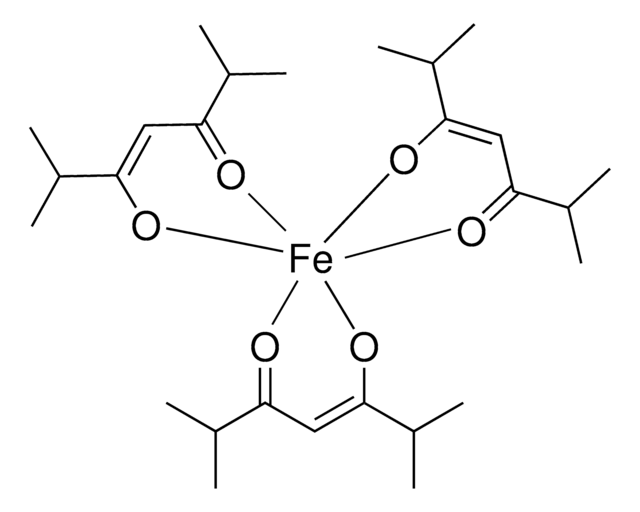
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)

![1-((3,5-Difluorophenyl)sulfonyl)bicyclo[2.1.0]pentane 95%](/deepweb/assets/sigmaaldrich/product/structures/307/973/0f3da9b2-92fc-4014-8832-b8613500f70a/640/0f3da9b2-92fc-4014-8832-b8613500f70a.png)

![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)

