A78608
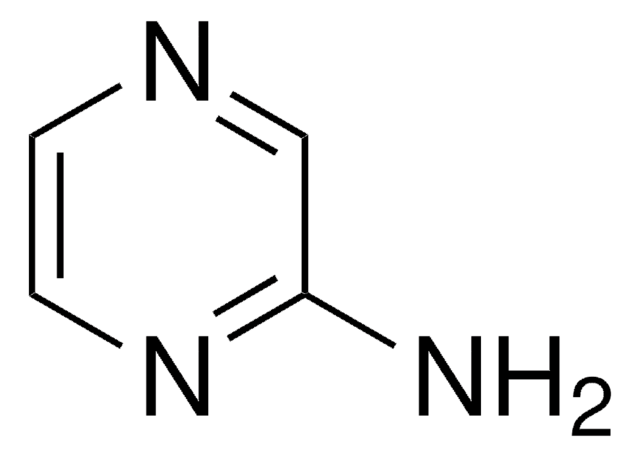
2-Aminopyrimidine
97%
Synonym(s):
2-Pyrimidinamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3
CAS Number:
Molecular Weight:
95.10
Beilstein/REAXYS Number:
107014
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
powder
mp
122-126 °C (lit.)
SMILES string
Nc1ncccn1
InChI
1S/C4H5N3/c5-4-6-2-1-3-7-4/h1-3H,(H2,5,6,7)
InChI key
LJXQPZWIHJMPQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kamaljit Singh et al.
European journal of medicinal chemistry, 52, 82-97 (2012-03-31)
2-Aminopyrimidine based 4-aminoquinolines were synthesized using an efficacious protocol. Some of the compounds showed in vitro anti-plasmodial activity against drug-sensitive CQ(S) (3D7) and drug-resistant CQ(R) (K1) strains of Plasmodium falciparum in the nM range. In particular, 5-isopropyloxycarbonyl-6-methyl-4-(2-nitrophenyl)-2-[(7-chloroquinolin-4-ylamino)butylamino] pyrimidine depicted the lowest
Nicholas R Perl et al.
Journal of the American Chemical Society, 132(6), 1802-1803 (2010-01-26)
A convergent synthesis of (-)-crambidine is reported. The sequence capitalizes on two novel key transformations, including a [4+2] annulation of thioimidates with vinyl carbodiimides and an alkyne hydroamination employing 2-aminopyrimidine nucleophiles.
Ye-Xiang Xie et al.
The Journal of organic chemistry, 71(21), 8324-8327 (2006-10-10)
Efficient and solvent-free copper-catalyzed N-arylations of imidazoles with aryl and heteroaryl halides have been demonstrated. In the presence of CuBr, 2-aminopyrimidine-4,6-diol, and TBAF (n-Bu4NF), a variety of imidazoles underwent the N-arylation reaction with aryl and heteroaryl halides smoothly in moderate
D S Ermolat'ev et al.
Molecular diversity, 15(2), 491-496 (2010-08-27)
An efficient microwave-assisted one-pot two-step protocol was developed for the construction of disubstituted 2-amino-1H-imidazoles. This process involves the sequential formation of 2,3-dihydro-2-hydroxyimidazo[1,2-a]pyrimidinium salts from readily available 2-aminopyrimidines and α-bromoketones, followed by cleavage of the pyrimidine ring with hydrazine.
Jinho Lee et al.
Bioorganic & medicinal chemistry letters, 21(14), 4203-4205 (2011-06-21)
A series of new 2-(2-aminopyrimidin-4-yl)phenol derivatives were synthesized as potential antitumor compounds. Substitution with pyrrolidine-3,4-diol at the 4-position of phenol provided potent inhibitory activity against CDK1 and CDK2. X-ray crystal structural studies were performed to account for the effect of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service