915793
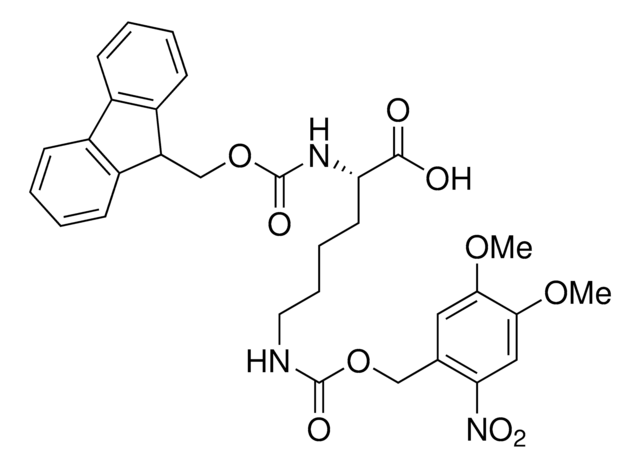
Methyl-o-nitropiperonyllysine
≥95%
Synonym(s):
N6-((1-(6-Nitrobenzo[d][1,3]dioxol-5-yl)ethoxy)carbonyl)-L-lysine, Light-triggered decaging Lys, Photo-controlled amino acid, Photocaged amino acid, Photocleavable lysine derivative, mNPK
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C16H21N3O8
CAS Number:
Molecular Weight:
383.35
MDL number:
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
Application
Methyl-o-nitropiperonyllysine (mNPK) trifluoroacetic acid is a photo-responsive unnatural amino acid (UAA) for spatiotemporal control of biological molecules or processes as reported by Kneuttinger et al. Irradiation with UV light decages the Lys amino acid, freeing the residue or protein for biological activity. Tools such as mNPK will find wide utility in light regulation of activity, allostery, and enzyme pathways.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Light Regulation of Enzyme Allostery through Photoresponsive Unnatural Amino Acids
Precise Photoremovable Perturbation of a Virus-Host Interaction
Genetic code expansion in the mouse brain
Genetically encoded optical activation of DNA recombination in human cells
Bioorthogonal Chemical Activation of Kinases in Living Systems
Precise Photoremovable Perturbation of a Virus-Host Interaction
Genetic code expansion in the mouse brain
Genetically encoded optical activation of DNA recombination in human cells
Bioorthogonal Chemical Activation of Kinases in Living Systems
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Arnaud Gautier et al.
Journal of the American Chemical Society, 133(7), 2124-2127 (2011-01-29)
We report a general strategy for creating protein kinases in mammalian cells that are poised for very rapid activation by light. By photoactivating a caged version of MEK1, we demonstrate the specific, rapid, and receptor independent activation of an artificial
James Hemphill et al.
Journal of the American Chemical Society, 135(36), 13433-13439 (2013-08-13)
Photocaging provides a method to spatially and temporally control biological function and gene expression with high resolution. Proteins can be photochemically controlled through the site-specific installation of caging groups on amino acid side chains that are essential for protein function.
Arnaud Gautier et al.
Journal of the American Chemical Society, 132(12), 4086-4088 (2010-03-12)
Precise photochemical control of protein function can be achieved through the site-specific introduction of caging groups. Chemical and enzymatic methods, including in vitro translation and chemical ligation, have been used to photocage proteins in vitro. These methods have been extended
Olivia S Walker et al.
Journal of the American Chemical Society, 138(3), 718-721 (2016-01-14)
Isocitrate dehydrogenase is mutated at a key active site arginine residue (Arg172 in IDH2) in many cancers, leading to the synthesis of the oncometabolite (R)-2-hydroxyglutarate (2HG). To investigate the early events following acquisition of this mutation in mammalian cells we
Hanna Engelke et al.
ACS synthetic biology, 3(10), 731-736 (2014-06-17)
Controlled manipulation of proteins and their function is important in almost all biological disciplines. Here, we demonstrate control of protein activity with light. We present two different applications-light-triggered transcription and light-triggered protease cleavage-both based on the same concept of protein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service