900664
Poly(D,L-lactide-co-glycolide)(50:50)-b-poly(ethylene glycol)
10k-2k
Synonym(s):
PLGA-b-PEG, PLGA-PEG
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
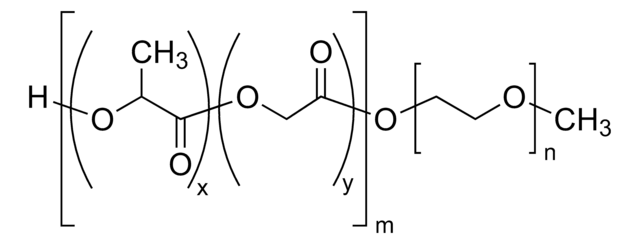
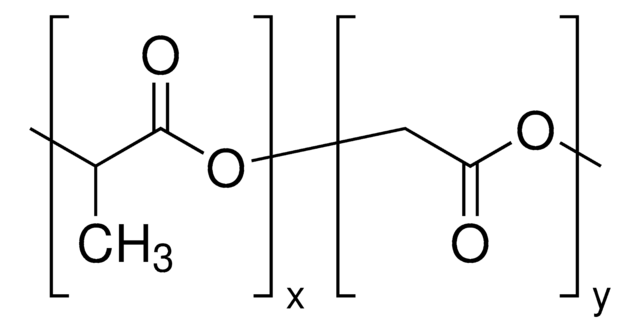
H[(C3H4O2)x(C2H2O2)y]mO[C2H4O]nCH3
UNSPSC Code:
12162002
NACRES:
NA.23
Recommended Products
Application
Biocompatible, amphiphilic block copolymer composed of a hydrophilic PEG block and a hydrophobic poly(D,L-lactide-co-glycolide) (PLGA) block. These materials have been used in control release and nanoparticle formulation for drug encapsulation and delivery applications. Well-defined materials with varying properties can be prepared by controlling the relative length of each polymer block. Hydroxyl termination allows for facile further chemical modification of these materials.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fabienne Danhier et al.
Journal of controlled release : official journal of the Controlled Release Society, 133(1), 11-17 (2008-10-28)
The purpose of this study was to develop Cremophor EL-free nanoparticles loaded with Paclitaxel (PTX), intended to be intravenously administered, able to improve the therapeutic index of the drug and devoid of the adverse effects of Cremophor EL. PTX-loaded PEGylated
Yihan Xu et al.
Journal of biomedical materials research. Part B, Applied biomaterials, 105(6), 1692-1716 (2016-04-22)
Poly (lactic-co-glycolic acid) (PLGA) copolymers have been broadly used in controlled drug release applications. Because these polymers are biodegradable, they provide an attractive option for drug delivery vehicles. There are a variety of material, processing, and physiological factors that impact
R Gref et al.
Science (New York, N.Y.), 263(5153), 1600-1603 (1994-03-18)
Injectable nanoparticulate carriers have important potential applications such as site-specific drug delivery or medical imaging. Conventional carriers, however, cannot generally be used because they are eliminated by the reticulo-endothelial system within seconds or minutes after intravenous injection. To address these
Miles A Miller et al.
Nature communications, 6, 8692-8692 (2015-10-28)
Therapeutic nanoparticles (TNPs) aim to deliver drugs more safely and effectively to cancers, yet clinical results have been unpredictable owing to limited in vivo understanding. Here we use single-cell imaging of intratumoral TNP pharmacokinetics and pharmacodynamics to better comprehend their
Articles
The development of drugs that target specific locations within the human body remains one of the greatest challenges in biomedicine today.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service