806382
Poly(N-isopropylacrylamide)
carboxylic acid terminated
Synonym(s):
NIPAM polymer, PNIPAM, functionalized polyNIPAM
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
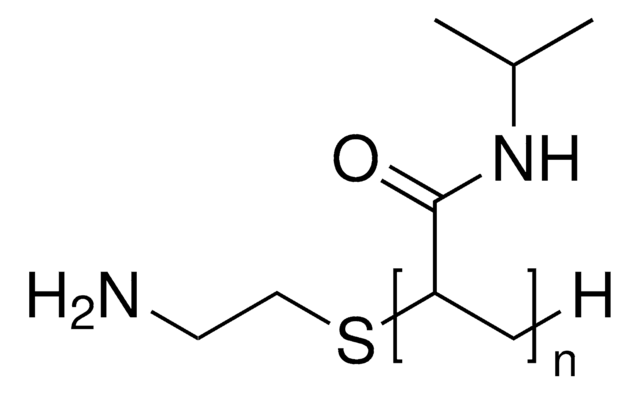
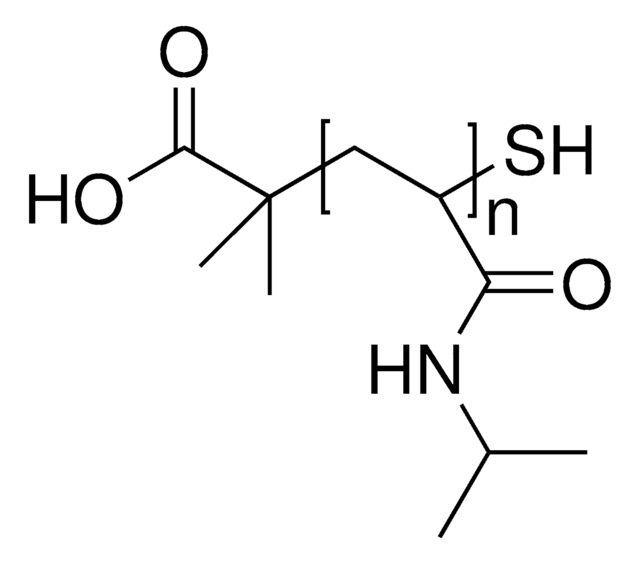
C4H7O2(C6H11NO)nH
UNSPSC Code:
12162002
NACRES:
NA.23
Recommended Products
form
powder
mol wt
average Mw 10,000
PDI
<1.3
storage temp.
2-8°C
Application
PolyNIPAM is a stimuli-responsive polymer. The low PDI means that the distribution of chain lengths is narrow which will typically lead to better reproducibility. This polymer for development of thermosensitive coated micro/nano materials including thermoresponsive polymeric drug delivery systems. The carboxylic acid functional group can be used to conjugate a variety of biomolecules to the polymer chain.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ravin Narain et al.
Langmuir : the ACS journal of surfaces and colloids, 23(11), 6299-6304 (2007-04-25)
We describe here the synthesis of 10 nm, monodisperse, iron oxide nanoparticles that we have coated with temperature-sensitive, biotinylated p(NIPAAm) (b-PNIPAAm). The PNIPAAm was prepared by the reversible addition fragmentation chain transfer polymerization (RAFT), and one end was biotinylated with
W L Hinrichs et al.
Journal of controlled release : official journal of the Controlled Release Society, 60(2-3), 249-259 (1999-07-30)
Copolymers of 2-(dimethylamino) ethyl methacrylate (DMAEMA) and N-isopropylacryl amide (NIPAAm) of various monomer ratios and molecular weights were evaluated as carrier systems for DNA delivery. All copolymers, even with a low DMAEMA content of 15 mol%, were able to bind
J E Chung et al.
Journal of controlled release : official journal of the Controlled Release Society, 62(1-2), 115-127 (1999-10-16)
To achieve a combination of spatial specificity in a passive manner with a stimuli-responsive targeting mechanism, a temperature-responsive polymeric micelle is prepared using block copolymers of (poly(N-isopropylacrylamide-b-butylmethacrylate) (PIPAAm-PBMA)). The micelle inner core formed by self-aggregates of PBMA segments successfully loaded
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service