★★★★★ No rating value Be the first to write a review . This action will open a modal dialog.
▼ Rating Filter by Rating
Filter by Rating 1 star 2 stars 3 stars 4 stars 5 stars
▼ Locale Filter by Locale
Filter by Locale sq العربية հայերեն Euskara Беларуская мова босански Български език Català 中文 Hrvatski jezik Čeština Dansk Nederlands English Eesti Suomi Français ქართული Deutsch Ελληνικά עברית Magyar Íslenska Indonesian Italiano 日本語 Қазақ тілі 한국어 Кыргызча Latviešu valoda Lietuvių kalba mk هاس ملايو Монгол хэл Norsk Język polski Português Limba română Русский Cрпски језик Slovenčina Slovenski jezik Español Svenska ไทย Türkçe Українська мова Việt Nam English (United States)
Active Filters Active Filters 1 star Remove Filter 1 star ✘ Active Filters 2 stars Remove Filter 2 stars ✘ Active Filters 3 stars Remove Filter 3 stars ✘ Active Filters 4 stars Remove Filter 4 stars ✘ Active Filters 5 stars Remove Filter 5 stars ✘ Active Filters sq Remove Filter sq ✘ Active Filters العربية Remove Filter العربية ✘ Active Filters հայերեն Remove Filter հայերեն ✘ Active Filters Euskara Remove Filter Euskara ✘ Active Filters Беларуская мова Remove Filter Беларуская мова ✘ Active Filters босански Remove Filter босански ✘ Active Filters Български език Remove Filter Български език ✘ Active Filters Català Remove Filter Català ✘ Active Filters 中文 Remove Filter 中文 ✘ Active Filters Hrvatski jezik Remove Filter Hrvatski jezik ✘ Active Filters Čeština Remove Filter Čeština ✘ Active Filters Dansk Remove Filter Dansk ✘ Active Filters Nederlands Remove Filter Nederlands ✘ Active Filters English Remove Filter English ✘ Active Filters Eesti Remove Filter Eesti ✘ Active Filters Suomi Remove Filter Suomi ✘ Active Filters Français Remove Filter Français ✘ Active Filters ქართული Remove Filter ქართული ✘ Active Filters Deutsch Remove Filter Deutsch ✘ Active Filters Ελληνικά Remove Filter Ελληνικά ✘ Active Filters עברית Remove Filter עברית ✘ Active Filters Magyar Remove Filter Magyar ✘ Active Filters Íslenska Remove Filter Íslenska ✘ Active Filters Indonesian Remove Filter Indonesian ✘ Active Filters Italiano Remove Filter Italiano ✘ Active Filters 日本語 Remove Filter 日本語 ✘ Active Filters Қазақ тілі Remove Filter Қазақ тілі ✘ Active Filters 한국어 Remove Filter 한국어 ✘ Active Filters Кыргызча Remove Filter Кыргызча ✘ Active Filters Latviešu valoda Remove Filter Latviešu valoda ✘ Active Filters Lietuvių kalba Remove Filter Lietuvių kalba ✘ Active Filters mk Remove Filter mk ✘ Active Filters هاس ملايو Remove Filter هاس ملايو ✘ Active Filters Монгол хэл Remove Filter Монгол хэл ✘ Active Filters Norsk Remove Filter Norsk ✘ Active Filters Język polski Remove Filter Język polski ✘ Active Filters Português Remove Filter Português ✘ Active Filters Limba română Remove Filter Limba română ✘ Active Filters Русский Remove Filter Русский ✘ Active Filters Cрпски језик Remove Filter Cрпски језик ✘ Active Filters Slovenčina Remove Filter Slovenčina ✘ Active Filters Slovenski jezik Remove Filter Slovenski jezik ✘ Active Filters Español Remove Filter Español ✘ Active Filters Svenska Remove Filter Svenska ✘ Active Filters ไทย Remove Filter ไทย ✘ Active Filters Türkçe Remove Filter Türkçe ✘ Active Filters Українська мова Remove Filter Українська мова ✘ Active Filters Việt Nam Remove Filter Việt Nam ✘ Active Filters English (United States) Remove Filter English (United States) ✘ Clear All ✘ Clear All Filters
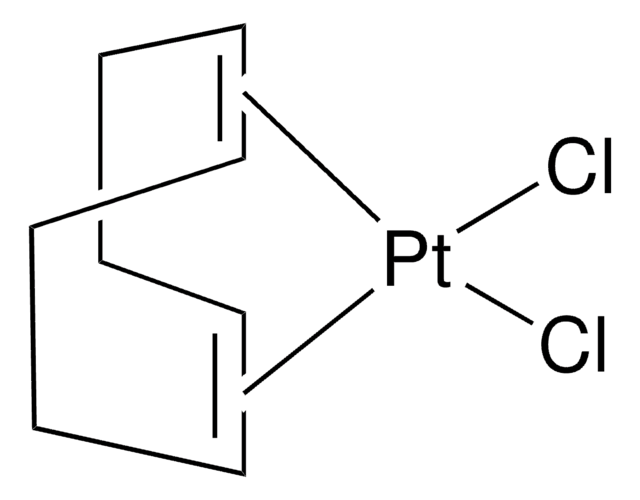




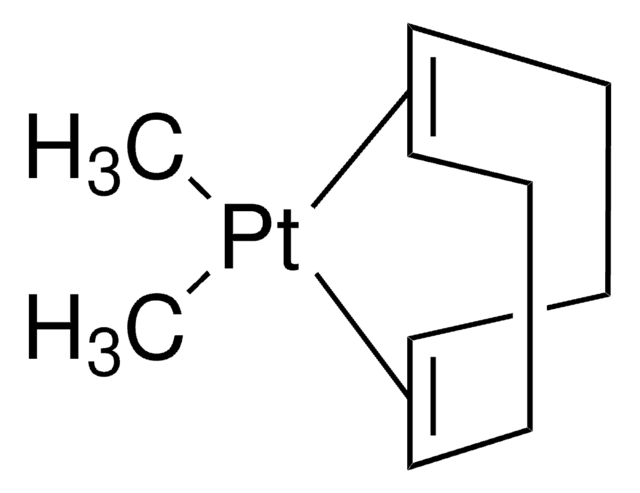


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)
